Abstract
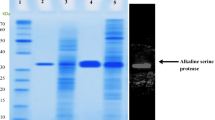
The alkaline protease structural gene (ALP1 gene) was isolated from both the genomic DNA and cDNA of Aureobasidium pullulans 10 by inverse PCR and RT-PCR. An open reading frame of 1248 bp encoding a 415 amino-acid protein with calculated molecular weight of 42.9 kDa was characterized. The gene contained two introns, which had 54 bp and 50 bp, respectively. The promoter of ALP1 gene was located from -62 to -112 and had two CCAAT boxes and one TATA box. The terminator of ALP1gene contained the sequence with a hairpin structure (AAAAAGTT TGGTTTTT). The protein sequence deduced from ALP1 gene exhibited 55.24%, 50.35%, and 31.68% identity with alkaline proteases from Aspergillus fumigatus, Acremonium chrysogenum, and Yarrowia lipolytica, respectively. The protein was found to have the conserved serine active site and histidine active site of serine proteases in the subtilisin family. The recombinant A. pullulans alkaline protease produced in Y. lipolytica formed clear zones on the double plates with 2% casein and alkaline protease activity in the supernatant of the recombinant Y. lipolytica culture was detected, suggesting that the cloned ALP1 gene is expressed in Y. lipolytica and the expressed alkaline protease is secreted into the medium.




Similar content being viewed by others
References
Anwar A, Saleemuddin M (1998) Alkaline protease: a review. Bioresour Technol 64:175–183
Barth G, Gaillardin C (1997) Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol Rev 19:219–237
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–253
Chi ZM, Liu ZQ, Gong F, Li HF (2006) Marine yeasts and their applications in mariculture. J Ocean Univer China 3:243–247
Chi ZM, Ma CL, Wang P, Li HF (2007) Optimization of medium and cultivation conditions for alkaline protease production by the marine yeast Aureobasidium pullulans. Bioresour Technol 98:534–538
Donaghy JA, McKay AM (1993) Production and properties of alkaline protease by Aureobasidium pullulans. J Appl Bacteriol 4:662–666
Inamura H, Nakai T, Muroga K (1985) An extracellular protease produced by Vibrio anguillarum. Bull Jpn Soc Sci Fish 51:1915–1920
Jolivalt C, Madzak C, Brault A, Caminade E, Malosse C, Mougin C (2005) Expression of laccase lllb from the white-rot fungus Trametes versicolor in the yeast Yarrowia lipolytica for environmental applications. Microbiol Biotechnol 66:450–456
Kumar CG, Takagi H (1999) Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol Adv 17:561–594
Liiv L, Parn P, Alamae T (2001) Cloning of maltase gene from a methylotrophic yeast, Hansenula polymorpha. Gene 265:77–85
Ma CL, Ni XM, Chi ZM, Ma LY, Gao LM (2007) Purification and characterization of an alkaline protease from the marine yeast Aureobasidium pullulans for bioactive peptide production from different sources. Mar Biotechnol 9:343–351
Madzak C, Gaillardin C, Beckerich JM (2004) Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica: a review. J Biotechnol 109:63–81
Matoba S, Fukuyama J, Wing RA, Ogrydziak DM (1988) Intracellular precursors and secretion of alkaline extracellular protease of Yarrowia lipolytica. Mole Cell Biol 8:4904–4916
Ogrydziak DM (1993) Yeast extracellular proteases. Crit Rev Biotechnol 13:1–55
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mole Biol Rev 62:597–635
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. 2nd edn. Cold Spring Harbor Laboratory Press, Beijing, pp. 367–370 (Chinese translating ed.)
Thompson JD, Higgins DD, Gibson JJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4688
Tobe S, Takami T, Ikeda S, Horikoshi K (1976) Production of some enzymatic properties of alkaline protease of Candida lipolytica. Agric Biol Chem 40:1087–1092
Xuan JM, Fournier P, Gaillardin C (1988) Cloning of the LYS5 gene encoding saccharopine dehydrogenase. Curr Genet 14:15–21
Acknowledgements
This research was supported by Hi-Tech Research and Development Program of China (863), grant no. 2006AA09Z403.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ni, X., Chi, Z., Ma, C. et al. Cloning, Characterization, and Expression of the Gene Encoding Alkaline Protease in the Marine Yeast Aureobasidium pullulans 10. Mar Biotechnol 10, 319–327 (2008). https://doi.org/10.1007/s10126-007-9067-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-007-9067-4