Abstract
Gastric cancer (GC) is one of the most common malignancies in China and is associated with high mortality. The occurrence and development of gastric cancer are related to genetic and environmental factors. Focal adhesion kinase (FAK) is a cytoplasmic nonreceptor protein tyrosine kinase that is activated by the extracellular matrix and growth factors. FAK is highly expressed in cancer and promotes its development by regulating cancer cell proliferation, migration, and angiogenesis. The expression of IL-8 is increased in many types of malignant tumor cells and is linked to their proliferation, migration, invasion, angiogenesis, and EMT. In this study, we found FAK to be essential for the proliferation, migration, and peritoneal metastasis of gastric cancer cells. To examine the molecular regulatory mechanisms of FAK in the peritoneal dissemination of gastric cancer, we performed RNA-seq analysis of MKN-45-FAK−/− and MKN45 cells and demonstrated that IL-8 was downregulated in FAK-deficient cells. Conversely, we confirmed that IL-8 activates FAK activity. We established that IL-8 promotes the proliferation, colony formation, and migration of gastric cancer cells that are partially mediated by FAK. Thus, we propose that an IL-8-FAK-IL-8 positive feedback loop effects the proliferation and migration of gastric cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most frequent malignancies in China and has a high mortality rate [1]. Peritoneal dissemination is the most common form of metastasis and the worst state of progression in gastric cancer [2, 3]. Helicobacter pylori infection, genetic susceptibility of the host, and environmental factors are suspected to cause the occurrence and progression of gastric cancer [4]. Dysregulation of multiple signaling pathways, due to the accumulation of genetic and epigenetic alterations in oncogenes and tumor suppressor genes, disrupts the cell cycle and the balance between cell proliferation and cell death [4]. However, the underlying mechanism of gastric cancer and its peritoneal dissemination is unknown. Further examination of the molecular mechanisms of gastric cancer is essential to identify new targets for tumor therapy.
Focal adhesion kinase (FAK) is a cytoplasmic nonreceptor protein tyrosine kinase that is activated by the extracellular matrix and growth factors [5]. FAK was first isolated in v-Src-transformed cells and was shown to localize to focal adhesions [6]. FAK is upregulated in various cancers, including gastric cancer [7,8,9,10,11]. FAK mRNA and protein are overexpressed in gastric cancer, correlating with tumor invasion and metastasis [12, 13]. Overexpression of FAK integrates signals from cell surface receptors to regulate adhesion, survival, migration, metastasis, invasion, lymphogenesis, and angiogenesis, all of which are canonical functions of FAK in cancer cells [14].
Interleukin-8(IL-8), also known as CXCL8, is a member of the CXC family of chemokines [15]. This widely expressed cytokine mediates cellular inflammatory responses and regulates autoimmune disease [16]. By interacting with CXCR1 and CXCR2, IL-8 activates the coupled G protein, which then induces signaling molecules to regulate gene expression; cell proliferation, differentiation, metabolism, and movement; and angiogenesis [16]. IL-8 is upregulated in many types of malignant tumor cells, which is linked to the proliferation, migration, invasion, angiogenesis, and EMT of tumor cells [17].
In this study, we found that FAK is essential for the peritoneal metastasis of gastric cancer. To examine the regulatory molecular mechanisms of FAK in the peritoneal dissemination of gastric cancer, we performed RNA-seq analysis of FAK-knockout and wild-type MKN45 cells and observed that IL-8 was downregulated in FAK-deficient cells. We further confirmed that IL-8 activates FAK activity in MKN45 cells and established that IL-8 promotes the proliferation and migration of gastric cancer cells that are partially mediated by FAK. Thus, we propose that an IL-8–FAK–IL-8 positive feedback loop promotes the proliferation and migration of gastric cancer cells.
Materials and methods
Cells and cell culture
The human gastric cancer cells MKN45, NUGC3, and NCIN87 were used. MKN45 and NCIN87 cells were obtained from the Cancer Hospital Chinese Academy of Medical Sciences, and NUGC3 cells were acquired from Nanjing Cobioer Biotechnology CO., LTD. The FAK-knockout gastric cancer cell line, termed MKN-45-FAK−/−, was established by our laboratory. All cell lines were grown in RPMI1640 (Biological Industries) that was supplemented with 10% fetal bovine serum (Biological Industries) and 1% penicillin/streptomycin (Gibco) and incubated at 37 °C in a humidified atmosphere with 5% CO2.
Reagents
The FAK inhibitor, TAE226, (Cat#HY-13203, Molecular formula: C23H25ClN6O3, molecular weight: 468.94) was purchased from Med Chem Express. The recombinant human chemokine IL-8 (Cat#208-IL-010/CF) was obtained from R&D systems. Phosphate-buffered saline (02-024-1ACS), RPMI1640 (Cat#01-100-1ACS) and HyClone (Cat#04-001-01ACS) were purchased from Biological Industries. Penicillin–streptomycin solution (FG101-01) and 0.25% trypsin (Cat#FG301-01) were purchased from Trans Gen Biotech. Fibronectin (ECM001) was purchased from Millipore. BSA (Cat#PC001) was purchased from Solarbio. CXCR1 (Cat#MAB330) and CXCR2 (Cat#MAB331) blocking antibodies were purchased from R&D systems. Anti-FAK (Cat#05-537) was purchased from Millipore. β-Actin (Cat# A5441) was purchased from Sigma. Anti-p-FAK (Tyr397) (Cat#700255) was purchased from Invitrogen. Goat anti-rabbit (Cat# ab136817) and goat anti-mouse (Cat# ab205719) were purchased from Abcam.
Generation of FAK-knockout vectors with CRISPR/Cas9
Guide RNA sequences for CRISPR/Cas9 were designed with the CC Top-CRISPR/Cas9 target online predictor (http://crispr.cos.uni-heidelberg.de). The targeting sgRNAs were selected and designed. Complementary oligo nucleotides for the guide RNAs (Table 1) were synthesized, annealed to double-strand DNA, and ligated into the BbsI (ThermoFisher) sites of the vector backbone (pX330-U6-Chimeric_BB-CBh-hSpCas9, Addgene) to generate the intact targeting plasmid. These constructs were confirmed through subcloning into pEASY-T1 vectors (Transgen) and sequencing using universal vector primers.
Genomic DNA extraction and surveyor digestion assay
Genomic DNA was extracted from cells 24–48 h post-transfection with the Wizard @ SV Genomic DNA Purification System following the manufacturer’s instructions. The genomic sequences that contained the target sequences of FAK gRNAs were PCR amplified using HiFi Super MixII (Transgen), 5′-GACTCCTTCCGCATAT-3′ forward primer, and 5′-AATTAGAACGCTGGTC-3′ reverse primer. The amplification program was as follows: 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 30 s, annealing at 54 °C for 30 s, and extension at 72 °C for 60 s, with a final extension of 10 min at 72 °C.
The PCR products were denatured and annealed for heteroduplex formation. Finally, the reaction product was incubated with 1 μl Surveyor Enhancer S and 2 μl Surveyor Nuclease S(IDT) at 42 °C for 1 h, and1/10 th the volume of Stop Buffer was added to terminate the reaction. The digested products were analyzed by 2% agarose gel electrophoresis.
Construction of FAK-knockout MKN45 cell line
The optimal sgRNA was selected according to the Surveyor digestion assay and then transfected into MKN45 cells as described. The cells were analyzed 24–48 h post-transfection by fluorescence microscopy (ZEISS) under the appropriate excitation filters. The harvested cells were washed twice, resuspended with 2 ml PBS, and analyzed on a BD flow cytometer to select individual cell colonies. Single cells were expanded into clones, and the cell colonies were subcultured after reaching 90% confluence in 96-well plates. The on-target effects were validated by Surveyor assay, DNA sequencing, and western blot following established protocols. Positive cell colonies were expanded and then cryopreserved. These cells were then thawed and cultured to reach a sufficient quantity in T75 flasks before being injected into nude mice. In addition, FAK-knockout MKN45 cells were also used to measure proliferation and migration.
CCK-8 assay
Cell proliferation after treatment with IL-8 was assessed using the Cell Counting Kit-8 (CCK-8) assay. Briefly, gastric cancer cells were seeded into 96-well plates at a density of 5 × 103 cells in 200 μl media per well and cultured for 24 h. Then, the media was removed, and fresh media that contained various concentrations of IL-8 (0, 5, 10, 20 ng/ml) and/or TAE226 (1 μmol/L) was added to the cells. BSA and DMSO alone were used as controls. After 48 h of treatment, 20 μl CCK8 reagent was added to the cells and incubated for 4 h away from light. The absorbance at 450 nm was measured on an INFINI M NAMO plate reader.
Cell migration assay
Cell migration was assessed using a cell culture insertion system (8 μM, Corning Life Sciences, MA, USA). After serum starvation for 8 h, 1.2 × 105 cells that were suspended in serum-free RPMI1640 were seeded in the upper chamber. The bottom chamber contained RPMI1640 with 10% FBS as a chemotactic factor to stimulate cell migration. All cells were allowed to migrate for 12 h, after which nonmigrating cells were removed by swabbing them from the top surface of the inserts; the cells that migrated toward the lower surfaces of the inserts were stained with 0.1% crystal violet for 30 min and quantified in five random fields for each membrane under a microscope (ZEISS).
RNA sequencing
MKN45 and MKN-45-FAK−/− cells were cultured in large dishes, after which 1 × 108 cells were collected and washed in PBS, and RNA was extracted using an RNA extraction kit. RNA-Seq was performed by BGI Technology Co., LTD.
qPCR analysis
Quantitative-time PCR (qPCR) was performed to determine the levels of IL-8 mRNA in MKN45; MKN-45-FAK−/−; andTAE226-treatedMKN45, NUGC3, and NCIN87 cells. MKN45 and MKN-45-FAK−/− cells were cultured, and total RNA was extracted using RNAiso Plus reagent according to the manufacturer's instructions (TaKaRa Co. Ltd., Dalian, China). MKN45, NUGC3, and NCIN87 cells were treated with various concentrations (0, 1, 5 μmol/L) of TAE226 for 24 h, and total RNA was prepared from untreated and treated cells.
Total cellular RNA was reverse-transcribed with oligo (dT)12–18 primer using Easy Script One-Step gDNA Removal and cDNA Synthesis SuperMix (Trans Gen Biotech Co. Ltd., Beijing, China). qPCR was performed using KAPA SYBP FAST qPCR kits (KAPA BIOSYSTEMS, Inc., Boston, MA, USA), optimized for the Light Cycler® 480 real-time PCR detection system (Roche Diagnostics International, Co. Ltd., Switzerland), according to the manufacturer’s instructions. All reactions were performed in 20 μl in triplicate. The program comprised an initial denaturation step at 95 °C for 5 min; 40 cycles of 95° C for 5 s, 54 °C for 30 s, and 72 °C for 20 s; and an extension of 72 °C for 10 min. 2−ΔΔCT values were calculated to determine expression levels, and the qPCR results were analyzed by Student’s t test to compare the expression between the untreated and treated groups. The relative amount of target gene mRNA was normalized to β-ACTIN. Primers for IL-8 and reference genes are listed in Table 2. The PCR generated 155-bp and 290-bp fragments of the IL-8 and β-actin genes, respectively.
Western blot
Cells were treated with DMSO or TAE226 at various concentrations for 48 h. Pretreated cells and FAK-knockout MKN45 cells were harvested, washed with cold 1 × PBS, lysed in protein lysis buffer (Thermo Fisher) for 10 min on ice, and centrifuged at 12,000 rpm for 10 min at 4 °C. The total protein concentration was determined by BCA protein assay (Thermo Fisher). Equal amounts of protein samples were subjected to SDS-PAGE electrophoresis, transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad), and incubated with primary antibodies overnight at 4 °C. After three washes in Tris-buffered saline with 1% Tween-20 (TBST), the blots were incubated with peroxidase-linked secondary antibodies at room temperature for1 h and washed three times in TBST. The bands were developed by chemiluminescence (Thermo Fisher). The antibodies that were used for the western blot are described above.
ELISA
Equal numbers of FAK-knockout (MKN-45-FAK−/−) and wild-type gastric cancer cells (MKN45) were seeded in six-well plates and incubated until 80% confluence. Cell culture supernatants were collected to measure IL-8 using a human IL-8 ELISA kit (Neobioscience technology Co. Ltd., Shenzhen, China) according to the manufacturer’s instructions. Absorbance at 450 nm and 630 nm was read on a Varioskan Flash Multimode Reader (Thermo Fisher Scientific, Pittsburgh, PA, USA). All measurements were performed in quintuplicate or triplicate, and the mean value of three independent measurements was used for the statistical analysis.
Immunofluorescent staining
Gastric cancer cells were seeded on slides in six-well plates and incubated overnight, after which the cells were washed with PBS and fixed with 4% paraformaldehyde for 15 min. Then, the cells were treated with Triton X-100 for 10 min and blocked with 1% BSA for 1 h. Next, the cells were incubated with primary antibodies against CXCR1 and CXCR2 overnight at 4 °C, washed with PBS, and incubated with FITC-labeled goat anti-mouse IgG for 1 h at room temperature. Nuclei were stained with DAPI. Finally, the slides were mounted with Glycerol Jelly Mounting Medium and examined under a laser-scanning confocal microscope (NIKON A1R, Nikon Corp., Tokyo, Japan).
Colony formation assay
MKN45, NUGC3, and NCIN87 cells were seeded in six-well plates at 100, 100, and 500 cells per well, respectively, and cultured for 24 h. Then, the media was removed, and fresh media that contained IL-8 (0, 10 ng/ml) was added. The media for these respective treatments was changed every 4 days. After 14 days, the cells were washed twice with PBS, fixed in 4% paraformaldehyde for 15 min, and stained with 0.1% crystal violet dye for 15 min. After being washed with distilled water and dried, the clones were photographed, counted, and analyzed.
CXCR1 and CXCR2 antibody blocking experiment
MKN45 cells were seeded in six-well plates at 30 × 104cells in 2 ml media per well and cultured for 24 h. Then, the media was removed, and fresh media without serum was added and incubated for 12 h. After serum starvation, fresh media that contained CXCR1-blocking or CXCR2-blocking antibody was added and incubated for 2 h, after which IL-8 (10 ng/ml) was added to the cells and incubated for 8 h. Finally, the cells were lysed, and total protein was collected for western blot analysis.
Animal experiments
All mouse experiments were performed according to Inner Mongolia University’s standard guidelines. Female BALB/c nu/nu mice, aged 4–6 weeks (Charles River Laboratories/Beijing Vital River Laboratory Animal Technology Co., Ltd), were maintained under pathogen-free conditions and used as a xenograft model of gastric cancer peritoneal dissemination. The mice were randomly assigned into four groups (n = 10 per group) before intraperitoneal injection of tumor cells. MKN45, syngeneic FAK-knockout cells (MKN-45-FAK−/−) and FAK-knockdown cells (MKN45-shFAK) were used to establish the peritoneal dissemination model. To generate intraperitoneal tumors, 3 × 106 cells were injected into the peritoneal cavity of the mice. These groups of mice—inoculated with MKN45 and MKN-45-FAK−/−cells/MKN45-shFAK, respectively—were killed 4 weeks after injection, when the degree of peritoneal dissemination was evaluated macroscopically. The mice were dissected, and the abdominal region was examined for visible tumors and photographed. Tumors that were greater than 0.5 mm in diameter were resected, measured, and prepared for histology. Tumor volumes were calculated as follows: tumor volume = length × width2 × 0.5. Two other groups of mice—inoculated with MKN45 and FAK-KO-MKN45 cells, respectively—were maintained to track their survival.
DNA constructs and in vitro transfection
FAK shRNA (shFAK) was designed and synthesized with the sequence 5′-aaGCCCTCAACCAGGGATTATGATTCAAGAGATCATAATCCCTGGTTGAGGGCtt-3′ to generate pGPU6/GFP/Neo-shFAK. The pGPU6/GFP/Neo-shFAK plasmids were transfected into MKN-45 cells using Lipofectamine TM2000 (Invitrogen, Carlsbad, New Mexico, USA) according the manufacturer’s instructions. Transfectants were selected with G418 (Hyclone Laboratories, Inc. Logan, UT, USA) for 48 h.
Statistical analyses
The statistical analyses were conducted using SPSS PASW Statistics for Windows, v18.0 (SPSS Inc.: Chicago, IL, USA). Normally distributed data were analyzed using standard parametric statistics and one-way ANOVA, followed by Tukey’s method. Data were expressed as mean ± SD. The results were presented as the average of at least three independent experiments. The western blot results were quantified on a Gel-Pro Analyzer 4.0 (Media Cybernetics, USA). Statistical significance was accepted at p ≤ 0.05.
Results
Construction of FAK-knockout cell line
To examine the function of FAK in the proliferation, migration, and peritoneal metastasis of gastric cancer cells in vitro and vivo, we used a stable FAK-knockout approach in MKN45 cells by CRISPR/Cas9. The FAK guide RNA targeted exon 1 of the FAK gene (Fig. 1A). Using an online sgRNA design program, four potential sgRNAs with the highest comprehensive score were selected, termed T3, T6, T7, and T14; their sequences are listed in Table 3. Oligonucleotides for the four guide RNAs were synthesized and cloned into the pX330 vector, and we confirmed the successful construction of CRISPR–Cas9 expression vectors by sequence assay. The resulting FAK-knockout vectors were termed pX330-FAK-T3, pX330-FAK-T6, pX330-FAK-T7, and pX330-FAK-T14 (Fig. 1B).
Construction of FAK-knockout cell line with CRISPR–Cas9 gene editing. A Potential sgRNAs exist in the first exon of FAK. B DNA sequence analysis shows correct linkage of pX330 and 4 sgRNAs. C Surveyor digestion assay. MKN45 cells were transfected with pX330-FAK-T3, pX330-FAK-T6, pX330-FAK-T7, and pX330-FAK-T14, and genomic PCR products were analyzed by Surveyor assay. MKN45 cells transfected with pX330-FAK-T6 were selected to isolate single colonies. D DNA sequence analysis shows the presence of the FAK insertion and deletion (indel) mutation in the target sites of clones #e, #f, and #c3 introduced by CRISPR–Cas9. E Mutation detected by the Surveyor system. Surveyor enzyme was used to confirm indel mutation in single clones transfected with pX330-FAK-T6. F Expression of FAK protein is significantly decreased in CRISPR–Cas9-mediated FAK-knockout cells. Western blot analysis confirmed that FAK knockout significantly decreases FAK in MKN45 cells compared with wild-type cells. β-Actin served as loading control. Histograms represent gray-level difference analysis of western blot. *p < 0.05, **p < 0.01
Next, we evaluated the gene-editing activity of each guide RNA by transfection of pX330-FAK-T3, pX330-FAK-T6, pX330-FAK-T7, and pX330-FAK-T14 separately, followed by Surveyor digestion assay. The Surveyor enzyme digested at mismatched sites, generating a wild-type band at approximately 530 bp and two mutantbands (200 bp and 330 bp). As shown in Fig. 1C, only cells that were transfected with pX330-FAK-T3 and pX330-FAK-T6 were cleaved at specific target sites and generated three correct bands; the lighter band appeared in cells with the latter plasmid. Thus, pX330-FAK-T6 was chosen for subsequent experiments to obtain FAK-knockout cell lines.
We used the CRISPR–Cas9 gene-editing system to generate FAK-knockout cell lines. Individual clones were isolated in 96-well plates from the transfected cells by flow cytometry and expanded in 48-well, 24-well, and 6-well plates. Then, we analyzed the sequences of the target DNA PCR products in these isolated lines and acquired three positive clones with indel mutations that were introduced into the genome (Fig. 1D). Next, the cleaved bands by Surveyor enzyme confirmed that insertion or deletion mutations (indel) were introduced into the genomes (Fig. 1E). Finally, by western blot, FAK expression was suppressed or abolished in FAK-knockout cell lines (Fig. 1F) (*p < 0.05, **p < 0.01). These results indicate that FAK-knockout cell lines were successfully generated by CRISPR–Cas9. Notably, clone #e was used in the follow-up experiments, based on its faster growth and higher target efficiency among the three double-stranded FAK-knockout clones. The resulting FAK-knockout (KO) gastric cancer cells were termed MKN45-FAK-KO (MKN-45-FAK−/−).
FAK-knockout suppresses the proliferation and migration of gastric cancer cells
Next, we determined whether FAK knockout affects cell proliferation by the CCK-8 assay. The optical density of control cells was 0.16, compared with 0.13 for MKN-45-FAK−/−cells (Fig. 2A) (***p < 0.001). The inhibition rate of proliferation in the FAK-knockout groups was 17%. These results show that gastric cancer cell proliferation is significantly suppressed in FAK-knockout MKN45 cells.
FAK knockout suppresses the proliferation and migration of gastric cancer cells. A FAK knockout suppresses the proliferation of gastric cancer cells. n = 3. B Micrographs of migration in MKN45 and FAK-knockout cells. C The number of migrated cells was counted and displayed in a bar graph; n = 3. Statistical significance was defined as p < 0.001(***), p < 0.01(**)
Recent studies have shown that FAK is important for cell migration in tumors. Thus, we examined whether knockout of FAK by CRISPR–Cas9 transfection affects the migratory activity of gastric cancer cells by Transwell assay. We noted significant inhibition of the migratory activities of FAK-knockout MKN45 cells. The average number of migrating control cells was 1183, decreasing to 544 in FAK-knockout cells, yielding a rate of inhibition of 54.03% (Fig. 2B, C) (**p < 0.01). These data demonstrate that gastric cancer cell migration is significantly inhibited in FAK-knockout MKN45 cells.
Decreased peritoneal disseminated tumors of gastric cancer after FAK knockout
The in vitro assay results showed that FAK knockout inhibits the proliferation and migration of gastric cancer cells, indicating the essential tumor-promoting functions of FAK in gastric cancer. Peritoneal dissemination is the most common form of metastasis and the worst state of progression of gastric cancer [2]. Thus, we studied whether the peritoneal dissemination of gastric cancer can be suppressed by FAK inhibition. Many disseminated nodules were established after the intraperitoneal injection of MKN45 cells, whereas few tumors were observed in mice that were injected with MKN45-FAK-KO cells (Fig. 3A, B). As shown in Fig. 3C, D, the total weight and volume of disseminated nodules were significantly lower in the group that received MKN45-FAK-KO versus MKN45 cells. MKN45 cells invaded many organs in the peritoneal cavity, including the peritoneum, spleen, liver, stomach, intestines, lung, and kidney, whereas FAK deficiency decreased the seeding and invasion tumor cells into these organs (Fig. 3E). FAK knockout prolonged the survival of disseminated tumor-bearing mice (Fig. 3F). Besides, we inhibited FAK expression in the MKN45 cells by using small hairpin RNA, and injected the FAK-knockdown cells into the mice peritoneal cavity. The results showed that peritoneal tumor metastasis was significantly reduced and volume and weight of tumors were also deduced in FAK-knockdown injecting group compared with the control (Fig. S1). These results indicate that FAK is essential for the peritoneal metastasis of gastric cancer cells.
Peritoneal disseminated tumors of gastric cancer are diminished after FAK knockout. Nude mice were injected intraperitoneally with MKN45 cells and MKN45-FAK-KO cells. A and B Macroscopic view of the peritoneal cavities of the two groups 4 weeks after injection. The mice were dissected, and the abdominal region was examined for visible tumors and photographed. C and D The weight and volumes of tumors were measured and calculated as tumor volume = length × width2 × 0.5. E MKN45 cells invaded many organs in the peritoneal cavity, including the peritoneum, spleen, liver, stomach, intestines, lung, and kidney, whereas FAK knockout decreased the seeding and invasion of tumor cells into these organs. F Survival of mice inoculated with MKN45 and FAK-KO-MKN45 cells. Error bars represent mean ± SD. Statistical significance was defined as p < 0.001(***)
IL-8 is downregulated in FAK-deficient cells
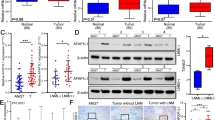
To examine the regulatory function of FAK in the progression of gastric cancer, we performed RNA sequencing (RNA-Seq) analysis of MKN-45-FAK−/− and MKN45 cells. MKN45 and MKN-45-FAK−/− cells were cultured in large dishes, after which 1 × 108 cells were collected and washed in PBS, and RNA was extracted. The results showed that 277 genes were significantly differentially expressed after FAK was knocked down, of which 94 were upregulated and 183 were downregulated. After GO functional classification and differentially expressed gene analysis (Fig. 4A, Fig. S2) of the differentially expressed genes, the expression of the chemokine CXCL8, also known as interleukin-8 (IL-8), was significantly lower in FAK-knockout versus wild-type cells. In addition, we utilized a web tool (cBioPortal: https://www.cbioportal.org/) to analyze TCGA data to explore the correlation between CXCL8 (IL-8) and PTK2 [18, 19]. The gene correlation results showed that there was a positive correlation between the expression of PTK2 and IL-8 (Fig. 4B). Also, IL-8 was significantly higher in the serum of gastric cancer patients compared to the healthy ones, as detected by the serum provided by the hospital (Fig. 4C). We performed a technical validation of the RNA-seq data by qPCR and found that IL-8 expression was lower in FAK-deficient cells, as expected (Fig. 4D). Also, by ELISA, the levels of IL-8 in the culture supernatants of FAK-knockout cells were lower compared with wild-type cells (Fig. 4E). These results validated the RNA-seq data and indicated that IL-8 expression was regulated by FAK.
IL-8 is down regulated in FAK-deficient cells and tumor tissues. A Volcano map of differentially expressed genes (DEGs) between MKN-45-FAK-/- and MKN45 cells. The X-axis represents log2 transformed difference multiplier values, and the Y-axis represents log10 transformed significance values. Red represents upregulated DEGs, blue represents downregulated DEGs, and gray represents non-DEG. B The correlation analysis between CXCL8 and PTK2. The stomach adenocarcinoma (TCGA, Nature 2014) data is utilized to analyze the correlation between CXCL8 and PTK2 by cBioPortal. The data contain 295 samples. C The expression of IL-8 in serum were measured by ELISA. Each point represents an individual, healthy: n = 41, gastric cancer patients: n = 57. ***p < 0.001. Error bar indicates SD. D mRNA levels of IL-8 in FAK-knockout cells were lower compared with wild-type cells. E IL-8 in the culture supernatants of FAK-knockout and wild-type cells. F IL-8 levels by qPCR in MKN45, NUGC3, and NCIN87 cells. G–I FAK, p-FAK, and IL-8 expression by western blot in MKN45, NUGC3, and NCIN87 cells after treatment with TAE226. Error bars represent mean ± SD. Statistical significance was defined as p < 0.001(***), p < 0.01(**), and p < 0.05(*)
To confirm that IL-8 expression is regulated by FAK in other gastric cancer cells, we treated three gastric cancer cell lines—MKN45, NUGC3, and NCIN87—with various concentrations of TAE226 (0, 1, 5 μmol/L) for 24 h and measured IL-8 by qPCR. IL-8 was also measured by western blot after treatment with several concentrations of TAE226 (0, 0.1, 0.5, 1, 5, 10 μmol/L) for 24 h. By qPCR and western blot, IL-8 expression was suppressed after FAK inhibition by TAE226 (Fig. 4F–I). These data show that the expression of IL-8 is suppressed after FAK inhibition in gastric cancer cells, indicating the regulatory function of FAK in IL-8 expression.
IL-8 promotes the proliferation, colony formation, and migration of gastric cancer cells
IL-8 has been proposed to be critical in regulating the progressive growth of human cancer cells [17]. The functions of IL-8 are mediated by CXCR1 and CXCR2, which are G protein-coupled receptors [20]. First, we measured the expression of CXCR1 and CXCR2 in MKN45, NUGC3, and NCIN87 cells by immunofluorescence. The results showed that CXCR1 and CXCR2 were detected in the three cell lines (Fig. 5A). To further study IL-8 in gastric cancer progression, we determined the effect of IL-8 on cell proliferation, colony formation, and migration in gastric cancer cells.
IL-8 promotes the proliferation, colony formation, and migration of gastric cancer cells. A Expression of CXCR1 and CXCR2 in MKN45, NUGC3, and NCIN87 cells by immunofluorescence. B Growth of by CCK-8 assay. C–E Colony formation assay in MKN45, NUGC3, and NCIN87 cells. F, G Cell migration assay of gastric cancer cells. Error bars represent mean ± SD. Statistical significance was defined as p < 0.001(***), p < 0.01(**), and p < 0.05(*)
We treated MKN45, NUGC3, and NCIN87 cells with various concentrations (0, 5, 10, 20 ng/ml) of recombinant IL-8 (rh-IL-8) for 48 h and measured growth by CCK8 assay. rh-IL-8 promoted the proliferation of the three gastric cancer lines. As shown in Fig. 5B, 10 ng/ml rh-IL-8 increased the growth of MKN45, NUGC3, and NCIN87 cells by 34%, 21%, and 6.5% increase, respectively. At 10 ng/ml, IL-8 had its maximum effect; thus, we chose this concentration for further experiments.
Next, we performed colony formation assay. As shown in Fig. 5C–E, rh-IL-8 (10 ng/ml) increased the clonogenicity of all gastric cancer lines—MKN45, NUGC3, and NCIN87 cells exhibited 18.9%, 29.6%, and 23.6% greater cell colony formation than the control cells, respectively. By cell migration assay, rh-IL-8 (10 ng/ml) increased the migration of MKN45 cells. As shown in Fig. 5F, G, the migration of MKN45 cells rose 24.3% after rh-IL-8 treatment.
IL-8-stimulated proliferation and migration of gastric cancer cells decrease after FAK inhibition
The results above indicated that the proliferation and migration of gastric cancer cells are promoted by IL8. The phosphorylation of FAK is induced by IL-8 [21]. Thus, we assumed that the effects of IL-8 on the proliferation and migration of gastric cancer cells are partially mediated by FAK. First, we assessed the phosphorylation of FAK in gastric cancer cells after treatment with IL-8 by western blot. As shown in Fig. 6A, IL-8 increased the phosphorylation of FAK in gastric cancer cells, indicating that IL8 activates the FAK pathway in gastric cancer cells. Then, we examined whether the phosphorylation of FAK due to IL-8 is mediated by CXCR1 or-CXCR2. As shown in Fig. 6B, the activity of FAK was inhibited after treatment with CXCR1-blocking and CRCR2-blocking antibodies, indicating that the phosphorylation of IL-8-induced FAK is mediated jointly by CXCR1 and CXCR2.
The IL-8-induced proliferation and migration of gastric cancer cells are diminished after FAK inhibition. A Phosphorylation of FAK in gastric cancer cells after treatment with IL-8 by western blot. B Phosphorylation of FAK after treatment with rh-IL-8 and CXCR1/CRCR2-blocking antibodies. C Cell proliferation byCCK8 assay. D Migration assay results. Error bars represent mean ± SD. Statistical significance was defined as p < 0.001(***), p < 0.01(**), and p < 0.05(*)
To determine whether the effects of IL8 on proliferation and migration in gastric cancer cells are mediated by FAK, CCK-8 assay and migration assay were performed to evaluate the effects of IL-8 and TAE226, separately and in combination. As shown in Fig. 6C, D, the proliferation and migration of IL-8-induced gastric cancer cells decreased after FAK inhibition. These results indicate that the promotion of proliferation and migration of gastric cancer cells by IL-8 are partially mediated by FAK.
Discussion
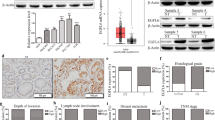
We have shown that FAK knockout decreases the size and levels of peritoneal disseminated gastric cancer tumors and prolongs the survival of disseminated tumor-bearing mice, indicating that FAK is essential for the peritoneal metastasis of gastric cancer. Further, the expression of IL-8 is regulated by FAK in gastric cancer cells, and IL-8 activates the FAK activity in turn, forming a positive feedback loop to promote the peritoneal dissemination of gastric cancer. Our data imply that the proliferation, migration, and peritoneal dissemination of gastric cancer cells are impeded by FAK knockout, implicating the tumor-promoting functions of FAK in gastric cancer. We have demonstrated that the expression of IL-8 is regulated by FAK in gastric cancer cells and established that IL-8 promotes the proliferation, colony formation, and migration of gastric cancer cells that are partially mediated by FAK. In addition, the cells used in the experiment were from poorly differentiated types, and the degree of tumor differentiation is an important indicator of tumor therapy. It has been shown that in gastric cancer, FAK expression was associated with increased tumor stage and decreased differentiation degree [22]. However, there are several reports showing the opposite results about the relationship between FAK expression and tumor differentiation. FAK expression was stronger in poorly differentiated laryngeal squamous carcinoma and esophageal squamous cell carcinoma [23, 24]; Ohta et al. also reported that reduced expression of FAK in intrahepatic cholangiocarcinoma is associated with poor tumor differentiation [25]; Murata et al. also reported that expressions of FAK and P-FAK increased as the degree of cell differentiation became higher in colorectal and esophageal carcinomas [26]. We also used UALCAN (http://ualcan.path.uab.edu/) to analyze TCGA data [27, 28], and the results showed that except in liver hepatocellular carcinoma, the expression of PTK2 is increased with the decrease of tumor cell differentiation grade. No significant relationship could be found among gastric cancer, esophageal carcinoma (ESCA) and head and neck squamous cell carcinoma (HNSC), which may be due to the small sample size (Supplementary Fig. 3). Therefore, we consider that the relationship between FAK and the grade of tumor differentiation is still unclear, and more experiments may be needed to explore and verify it.
Peritoneal dissemination is one of the worst states of gastric cancer, the progression of which is a complex multistep process, divided into several basic stages: (1) the detachment of gastric cancer cells from the primary tumor; (2) the survival of free cancer cells in the microenvironment of the abdominal cavity; (3) the attachment of free cancer cells to the peritoneal surface and invasion of the basement membrane in the peritoneum; and (4) the growth and angiogenesis of cancer cells, leading to peritoneal metastasis [3]. Many adhesion molecules, matrix proteases, motility factors, and angiogenic factors in the tumor microenvironment are involved in this process, but the underlying mechanisms of peritoneal dissemination remain unknown. Our previous results have shown that oral administration of an FAK inhibitor reduces the size of peritoneal disseminated tumors in colorectal cancer and prolongs survival in tumor-bearing mice [29]. Our current study also implies that FAK is essential for the development of the peritoneal dissemination of gastric cancer.
FAK is essential for the assembly and disassembly of focal adhesions (FAs) [30]. As a nonreceptor protein tyrosine kinase that localizes to FAs, FAK is activated by integrins, growth factor receptors, G protein-coupled receptors, and cytokine receptors [31]. In cancer cells, overexpressed FAK integrates signals from cell surface receptors to regulate adhesion, survival, migration, metastasis, invasion, lymphomagenesis, and angiogenesis, all of which are canonical functions of FAK in cancer cells [30,31,32]. Thus, inhibition or knockout of FAK could decrease the peritoneal dissemination of colorectal and gastric cancer, which due to the canonical roles of FAK in cancer cells. However, our data imply that the expression of IL-8 is regulated by FAK and that IL-8 activates FAK in turn, constituting a pathway that promotes the peritoneal dissemination of gastric cancer.
Some reports have described the roles of FAK in inflammation and the immune response. FAK is involved in the production of inflammatory mediators that are induced by protein I/II, a cell wall component from streptococci, in human monocytes, epithelial cells, endothelial cells, and fibroblasts [5, 33]. LPS-induced cytokine release also depends on FAK, suggesting that FAK has a general function in cytokine expression [34]. FAK signaling is important for TNF-α-induced IL-6 mRNA and protein expression in breast, lung, prostate, and neural tumor cells [35]. Funakoshi-Tago et al. reported the function of FAK in the NF-κB activation pathway in regulating various inflammatory and immune responses [36]. Nuclear FAK could promote the evasion of antitumor immunity by controlling chemokine transcription [37]. Our data show that the expression of IL-8 is regulated by FAK in gastric cancer cells, implicating FAK in inflammatory factor production and suggesting a novel cancer-promoting mechanism in gastric cancer.
In addition to the classical functions of IL-8 in the inflammatory response, IL-8 has been demonstrated to be closely related to the proliferation, EMT, migration, and invasion of tumor cells [17, 38]. After binding to CXCR1 or CXCR2, IL-8 activates the G protein, activating the PLC–PKC, PI3K–AKT, Src, and FAK pathways, promoting the proliferation, survival, angiogenesis, EMT, migration, and invasion of tumor cells [39]. Our data imply that IL-8 activates FAK through CXCR1 and/or CXCR2 in gastric cancer cells and that the promotion of the proliferation and migration of gastric cancer cells by IL-8 are partially mediated by the FAK pathway. FAK is overexpressed in various cancers, including gastric cancer [40]. IL-8 is secreted by monocytes, macrophages, neutrophils, lymphocytes, vascular endothelial cells, and fibroblasts [12]. IL-8 is also produced by tumor cells, tumor-associated fibroblasts, and tumor-associated macrophages in the tumor microenvironment, which promote tumor growth and metastasis through autocrine and paracrine mechanisms [17, 38]. Thus, overexpressed FAK mediates the signals from IL-8 in the tumor microenvironment through CXCR1 and CXCR2 to promote the proliferation, survival, migration, and invasion of gastric cancer cells, leading to their peritoneal dissemination. In conclusion, we propose that the IL–8-FAK–IL-8 positive feedback loop promotes the development of the peritoneal dissemination of gastric cancer.
Data availability
The datasets in this study are available in the article file or can be obtained on request.
References
Gao K, Wu J. National trend of gastric cancer mortality in China (2003–2015): a population-based study. Cancer Commun (Lond). 2019;39(1):24. https://doi.org/10.1186/s40880-019-0372-x.
Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134(3):622–8. https://doi.org/10.1002/ijc.28373.
Kanda M, Kodera Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J Gastroenterol. 2016;22(30):6829–40. https://doi.org/10.3748/wjg.v22.i30.6829.
Berger H, Marques MS, Zietlow R, Meyer TF, Machado JC, Figueiredo C. Gastric cancer pathogenesis. Helicobacter. 2016;21(Suppl 1):34–8. https://doi.org/10.1111/hel.12338.
Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14(9):598–610. https://doi.org/10.1038/nrc3792.
Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89(11):5192–6. https://doi.org/10.1073/pnas.89.11.5192.
Golubovskaya VM, Kweh FA, Cance WG. Focal adhesion kinase and cancer. HistolHistopathol. 2009;24(4):503–10. https://doi.org/10.14670/HH-24.503.
Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342(8878):1024–5. https://doi.org/10.1016/0140-6736(93)92881-s.
Lark AL, Livasy CA, Calvo B, Caskey L, Moore DT, Yang X, et al. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin Cancer Res. 2003;9(1):215–22.
Lightfoot HM Jr, Lark A, Livasy CA, Moore DT, Cowan D, Dressler L, et al. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res Treat. 2004;88(2):109–16. https://doi.org/10.1007/s10549-004-1022-8.
Giaginis CT, Vgenopoulou S, Tsourouflis GS, Politi EN, Kouraklis GP, Theocharis SE. Expression and clinical significance of focal adhesion kinase in the two distinct histological types, intestinal and diffuse, of human gastric adenocarcinoma. Pathol Oncol Res. 2009;15(2):173–81. https://doi.org/10.1007/s12253-008-9120-2.
Lai IR, Chu PY, Lin HS, Liou JY, Jan YJ, Lee JC, et al. Phosphorylation of focal adhesion kinase at Tyr397 in gastric carcinomas and its clinical significance. Am J Pathol. 2010;177(4):1629–37. https://doi.org/10.2353/ajpath.2010.100172.
Park JH, Lee BL, Yoon J, Kim J, Kim MA, Yang HK, et al. Focal adhesion kinase (FAK) gene amplification and its clinical implications in gastric cancer. Hum Pathol. 2010;41(12):1664–73. https://doi.org/10.1016/j.humpath.2010.06.004.
Kleinschmidt EG, Schlaepfer DD. Focal adhesion kinase signaling in unexpected places. CurrOpin Cell Biol. 2017;45:24–30. https://doi.org/10.1016/j.ceb.2017.01.003.
Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9(1):9–23. https://doi.org/10.1016/s1359-6101(97)00022-1.
Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7(6):1543–88. https://doi.org/10.7150/thno.15625.
Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31. https://doi.org/10.1016/j.ctrv.2017.08.004.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. https://doi.org/10.1126/scisignal.2004088. (PMID: 23550210; PMCID: PMC4160307).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9. https://doi.org/10.1038/nature13480. (Epub 2014 Jul 23. PMID: 25079317; PMCID: PMC4170219).
Park SH, Das BB, Casagrande F, Tian Y, Nothnagel HJ, Chu M, et al. Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature. 2012;491(7426):779–83. https://doi.org/10.1038/nature11580.
Cohen-Hillel E, Yron I, Meshel T, Ben-Baruch A. Interleukin 8 and cell migration to inflammatory sites: the regulation of focal adhesion kinase under conditions of migratory desensitization. Isr Med Assoc J. 2007;9(8):579–83 (PMID: 17877062).
Luo Q, Zhang S, Zhang D, Yuan F, Chen X, Yang S. Expression of ASAP1 and FAK in gastric cancer and its clinicopathological significance. Oncol Lett. 2020;20(1):974–80. https://doi.org/10.3892/ol.2020.11612. (Epub 2020 May 13. PMID: 32566028; PMCID: PMC7285715).
Aronsohn MS, Brown HM, Hauptman G, Kornberg LJ. Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in squamous cell carcinoma of the larynx. Laryngoscope. 2003;113(11):1944–8. https://doi.org/10.1097/00005537-200311000-00017. (PMID: 14603053).
Miyazaki T, Kato H, Nakajima M, Sohda M, Fukai Y, Masuda N, et al. FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 2003;89(1):140–5. https://doi.org/10.1038/sj.bjc.6601050. (PMID: 12838315; PMCID: PMC2394235).
Ohta R, Yamashita Y, Taketomi A, Kitagawa D, Kuroda Y, Itoh S, et al. Reduced expression of focal adhesion kinase in intrahepatic cholangiocarcinoma is associated with poor tumor differentiation. Oncology. 2006;71(5–6):417–22. https://doi.org/10.1159/000107109. (Epub 2007 Aug 8. PMID: 17687194).
Murata T, Naomoto Y, Yamatsuji T, Okawa T, Shirakawa Y, Gunduz M, et al. Localization of FAK is related with colorectal carcinogenesis. Int J Oncol. 2008;32(4):791–6 (PMID: 18360706).
Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27. https://doi.org/10.1016/j.neo.2022.01.001. (Epub 2022 Jan 22. PMID: 35078134; PMCID: PMC8788199).
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–58. https://doi.org/10.1016/j.neo.2017.05.002. (Epub 2017 Jul 18. PMID: 28732212; PMCID: PMC5516091).
Hao HF, Takaoka M, Bao XH, Wang ZG, Tomono Y, Sakurama K, et al. Oral administration of FAK inhibitor TAE226 inhibits the progression of peritoneal dissemination of colorectal cancer. Biochem Biophys Res Commun. 2012;423(4):744–9. https://doi.org/10.1016/j.bbrc.2012.06.030.
Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123(Pt 7):1007–13. https://doi.org/10.1242/jcs.045112.
Yoon H, Dehart JP, Murphy JM, Lim ST. Understanding the roles of FAK in cancer: inhibitors, genetic models, and new insights. J HistochemCytochem. 2015;63(2):114–28. https://doi.org/10.1369/0022155414561498.
Golubovskaya VM. Targeting FAK in human cancer: from finding to first clinical trials. Front Biosci (Landmark Ed). 2014;19(4):687–706. https://doi.org/10.2741/4236.
Neff L, Zeisel M, Druet V, Takeda K, Klein JP, Sibilia J, et al. ERK 1/2- and JNKs-dependent synthesis of interleukins 6 and 8 by fibroblast-like synoviocytes stimulated with protein I/II, a modulin from oral streptococci, requires focal adhesion kinase. J Biol Chem. 2003;278(30):27721–8. https://doi.org/10.1074/jbc.M212065200.
Al-Okla S, Chatenay-Rivauday C, Klein JP, Wachsmann D. Involvement of alpha5beta1 integrins in interleukin 8 production induced by oral viridans streptococcal protein I/IIf in cultured endothelial cells. Cell Microbiol. 1999;1(2):157–68. https://doi.org/10.1046/j.1462-5822.1999.00016.x.
Zeisel MB, Druet VA, Sibilia J, Klein JP, Quesniaux V, Wachsmann D. Cross talk between MyD88 and focal adhesion kinase pathways. J Immunol. 2005;174(11):7393–7. https://doi.org/10.4049/jimmunol.174.11.7393.
Funakoshi-Tago M, Sonoda Y, Tanaka S, Hashimoto K, Tago K, Tominaga S, et al. Tumor necrosis factor-induced nuclear factor kappaB activation is impaired in focal adhesion kinase-deficient fibroblasts. J Biol Chem. 2003;278(31):29359–65. https://doi.org/10.1074/jbc.M213115200. (Epub 2003 May 14).
Schlaepfer DD, Hou S, Lim ST, Tomar A, Yu H, Lim Y, et al. Tumor necrosis factor-alpha stimulates focal adhesion kinase activity required for mitogen-activated kinase-associated interleukin 6 expression. J Biol Chem. 2007;282(24):17450–9. https://doi.org/10.1074/jbc.M610672200.
Serrels A, Lund T, Serrels B, Byron A, McPherson RC, von Kriegsheim A, et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015;163(1):160–73. https://doi.org/10.1016/j.cell.2015.09.001.
Sharma I, Singh A, Siraj F, Saxena S. IL-8/CXCR1/2 signalling promotes tumor cell proliferation, invasion and vascular mimicry in glioblastoma. J Biomed Sci. 2018;25(1):62. https://doi.org/10.1186/s12929-018-0464-y.
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, Wu K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61–71. https://doi.org/10.1016/j.cytogfr.2016.08.002.
Acknowledgements
We thank Associate Professor Xudong Guo for his generous help with animal experiments, and Dr. Xuan Wang and Ms. Xiaoyang Jia for their generous help in performing the laser-scanning confocal microscopy.
Funding
This work was supported by the Natural Sciences Foundation of China (NO. 31860309, 32160836), Science and Technology Major Project of Inner Mongolia Autonomous Region of China (No. 2020ZD15), Inner Mongolia Key Laboratory for Molecular Regulation of the Cell (NO. 2021PT0002).
Author information
Authors and Affiliations
Contributions
YM performed the experiments shown in Fig. 4, Fig. 5 and the all supplementary files. YF performed the experiments shown in Fig. 1, Fig. 2 and Fig. 3. XF performed the experiments shown in Fig. 6. QJ, XD and YW modified the manuscript and figures. ZW and HH designed the project and revised the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10120_2023_1384_MOESM1_ESM.tif
Supplementary file1 Supplementary Figure 1 Peritoneal disseminated tumors of gastric cancer are diminished after FAK knockdown. Expression of protein (A) and mRNA (B) of FAK in control MKN45 and pGPU6/GFP/Neo-shFAK-transfected MKN45. P<0.01 (**). n=3 independent experiments. Error bars indicate SD. (C) Nude mice were injected intraperitoneally with MKN45 cells and MKN45-shFAK cells. Macroscopic observations of the abdominal cavity of both groups were taken at defined time points 4 weeks after injection. Mice were dissected and the abdominal region was examined for visible tumors and photographed. (D) The weight and volume of tumors were measured and calculated according to the following equation. Tumor volume = length × width 2 × 0.5. Error bars represent the mean ± SD. statistical significance was defined as P<0.001 (***) (TIF 58097 KB)
10120_2023_1384_MOESM2_ESM.tif
Supplementary file2 Supplementary Figure 2 The transcriptome analysis. (A) Heatmap of the differentially expressed genes between MKN-45-FAK-/- and MKN45 cells. Red stripes in the figure represent high-expression genes, while blue stripes represent low-expression genes. (B) Significant enriched Gene Ontology (GO) terms between MKN-45-FAK-/- and MKN45 cells. (C) Pathway classification of differential genes. X-axis represents the proportion of genes and Y-axis represents KEGG functional classification. The genes were divided into seven branches according to the KEGG metabolic pathways involved: cellular processes, Environmental Information Processing, Genetic Information Processing, Human diseases (animals only), Metabolism, organic Systems Organismal Systems, and drug Development. (TIF 104778 KB)
10120_2023_1384_MOESM3_ESM.tif
Supplementary file3 Supplementary Figure 3 The different tumor grade expression level of FAK in STAD, ESCA, LIHC and HNSC. (A) The expression PTK2 in STAD is based on tumor grade. (B) The expression PTK2 in ESCA is based on tumor grade. (C) The expression PTK2 in LIHC based on tumor grade. (D) The expression PTK2 in HNSC is based on tumor grade. Data is resorted from (http://ualcan.path.uab.edu/). Grade1: Well differentiated (low grade); Grade2: Moderately differentiated (intermediate grade); Grade3: Poorly differentiated (high grade); Grade4: Undifferentiated (high grade). (TIF 69178 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Fu, Y., Fan, X. et al. FAK/IL-8 axis promotes the proliferation and migration of gastric cancer cells. Gastric Cancer 26, 528–541 (2023). https://doi.org/10.1007/s10120-023-01384-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-023-01384-3