Abstract
Background
Dual-targeted anti-HER2 therapy significantly improves outcomes in HER2-positive breast cancer and could be beneficial in other HER2-positive cancers. JACOB’s end-of study analyses aimed to evaluate the long-term efficacy and safety of pertuzumab plus trastuzumab and chemotherapy for previously untreated HER2-positive metastatic gastric or gastroesophageal junction cancer.
Methods
Eligible patients were randomized 1:1 to pertuzumab/placebo plus trastuzumab and chemotherapy every 3 weeks. Primary endpoint: overall survival (OS). Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), duration of response (DoR), and safety.
Results
The intention-to-treat population comprised 388 patients in the pertuzumab arm and 392 in the placebo arm. The safety population comprised 385 and 388 patients, respectively. Median follow-up was ≥ 44.4 months. Median OS was increased by 3.9 months (hazard ratio 0.85 [95% confidence intervals, 0.72–0.99]) and median PFS by 1.3 months (hazard ratio 0.73 [95% confidence intervals, 0.62–0.85]) in the pertuzumab vs. the placebo arm. ORR was numerically higher (57.0% vs. 48.6%) and median DoR 1.8 months longer with pertuzumab treatment. There was a trend for more favorable hazard ratios in certain subgroups related to HER2 amplification/overexpression. Safety was comparable between arms, except for serious and grade 3–5 adverse events, and any-grade diarrhea, which were more frequent with pertuzumab.
Conclusions
JACOB did not meet its primary endpoint. Nonetheless, the study continues to demonstrate some, albeit limited, evidence of treatment activity and an acceptable safety profile for pertuzumab plus trastuzumab and chemotherapy in previously untreated HER2-positive metastatic gastric or gastroesophageal junction cancer after long-term follow-up.
Trial registration NCT01774786; https://clinicaltrials.gov/ct2/show/NCT01774786.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Trastuzumab for Gastric Cancer (ToGA) study demonstrated significantly improved overall survival (OS) with the addition of trastuzumab to chemotherapy in patients with previously untreated HER2-positive locally advanced/metastatic gastric/gastroesophageal junction cancer (GC/GEJC) [1]. Adding pertuzumab to trastuzumab and chemotherapy has been shown to significantly improve clinical outcomes in patients with HER2-positive early and metastatic breast cancer (BC) and the combination has been approved for patients with HER2-positive colorectal cancer in Japan based on the results of the TRIUMPH study [2,3,4,5,6]; however, there are several differences in tumor biology between BC and GC, including a higher incidence of HER2 heterogeneity and incomplete membrane staining in GC [7]. JACOB was designed to assess the efficacy and safety of pertuzumab plus trastuzumab and chemotherapy in patients with previously untreated HER2-positive metastatic GC/GEJC [6]. Primary results showed that addition of pertuzumab did not significantly improve OS at ≥ 24.4 months median follow-up [8]. We report descriptive end-of-study results, including previously unreported biomarker results, at ≥ 44.4 months median follow-up.
Methods
Study design and patients
JACOB (NCT01774786) was a double-blind, placebo-controlled, randomized, multicenter, phase III trial. Details have been published previously [8].
Eligible patients with previously untreated HER2-positive (centrally assessed immunohistochemistry [IHC] 3 + or IHC 2 + /in situ hybridization [ISH]-positive) GC/GEJC (n = 780) were randomized 1:1 to intravenous pertuzumab (840 mg) / placebo plus trastuzumab (8 mg/kg loading, 6 mg/kg maintenance doses) and chemotherapy (intravenous cisplatin 80 mg/m2 plus capecitabine 1000 mg/m2 orally twice daily, or intravenous 5-flurouracil 800 mg/m2 every 24 h continuously for 120 h) every 3 weeks. Chemotherapy discontinuation during/before cycle 6 was allowed for progressive disease/unacceptable toxicity. Chemotherapy continuation post-cycle 6 was at the discretion of the patient and treating physician. Pertuzumab/placebo and trastuzumab were continued following chemotherapy completion until disease progression, unacceptable toxicity, or withdrawal from the study.
The primary endpoint was OS. Secondary endpoints included progression-free survival (PFS), objective response rate (ORR), duration of response (DoR), and safety. Exploratory endpoints included association of biomarkers with efficacy outcomes.
To assess HER2 heterogeneity further, subgroups were defined by percentages of stained cancer cells, per the following categories: focal staining: 0–29% of cells staining positive for HER2; heterogenous staining: 30–79% of cells staining positive for HER2; and homogenous staining: 80–100% of cells staining positive for HER2. Subgroups were also defined by the gene copy number; this term refers to the number of copies of the HER2 gene that were determined by an in situ hybridization test. An average of 6 copies of the HER2 gene per cell/nucleus was used as a cutoff for this analysis to signify gene amplification. Per the American Society of Clinical Oncology (ASCO) guidelines, a gene copy number of > 6 signifies true amplification, whereas 4–6 copies is considered an equivocal result [9].
Statistical analysis
Target sample sizes and power calculations for the primary analysis have been reported [8]. Analyses were performed with SAS (version 9.2 and 9.4; SAS Institute Inc., NC, USA). OS and PFS were analyzed in the intention-to-treat (ITT) population (all randomized patients regardless of whether they received study treatment) according to study arm allocation. ORR was assessed in patients in the ITT population with measurable disease at baseline; those with no tumor assessment data after baseline were classed as non-responders. Safety analyses were conducted in all patients who received at least one dose of study treatment and was analyzed according to arm allocation. The Kaplan–Meier method was used to estimate OS, PFS, and DoR, with 95% confidence intervals (CIs) calculated using the Brookmeyer–Crowley method. A stratified Cox proportional hazards regression model was used to estimate the hazard ratio (HR) between arms with 95% CIs (stratification factors: geographic region, HER2 status, previous gastrectomy). The proportion of patients who achieved an overall response was summarized and 95% CIs calculated using the Clopper–Pearson method. Adverse events (AEs) were graded per standard criteria. All analyses are descriptive.
Results
Patients
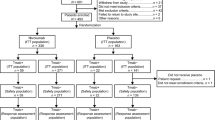
Of the 780 eligible patients enrolled between June 10, 2013 and January 12, 2016, 388 were randomized to the pertuzumab arm and 392 to the placebo arm (ITT population) [8]. The safety population comprised 385 and 388 patients in the pertuzumab and placebo arms, respectively (including one who received both treatments in error and was counted in the pertuzumab arm) [8]. Patient dispositions are shown in Fig. 1. The clinical cutoff for this analysis was January 24, 2020.
Efficacy
At a median follow-up of 46.1 and 44.4 months, respectively, there were 300 OS and 342 PFS events in the pertuzumab arm, and 319 OS and 353 PFS events in the placebo arm). Median OS increased by 3.9 months in the pertuzumab vs. placebo arm; a 15% reduction in risk of death (HR 0.85 [95% CI, 0.72–0.99]) (Table 1 and Fig. 2). Median PFS increased by 1.3 months in the pertuzumab vs. placebo arm; a 27% reduction in risk of progressive disease (HR 0.73 [95% CI, 0.62–0.85]) (Table 1). ORR was numerically higher (57.0% vs. 48.6%) and median DoR was 1.8 months longer in the pertuzumab vs. placebo arm (Table 1).
OS subgroup analyses
When OS was analyzed by baseline characteristics and stratification factors, HRs were similar to that observed in the ITT population for most subgroups (Fig. 3A). Similarly, no clear differences in HR were observed in biomarker subgroups, although there was a trend for more favorable HRs in certain subgroups related to HER2 amplification/overexpression (Fig. 3B). OS was longer for patients with a HER2 status of IHC 3 + vs. IHC 2 + /ISH-positive; with homogenous HER2 IHC staining patterns vs. heterogenous or focal staining patterns; and with higher HER2 copy numbers/mRNA levels in both arms (Fig. 3B). HER3 and phosphatase and tensin homolog (PTEN) did not show any association with OS (Fig. 3B).
OS by biomarker subgroups (ITT population). CI confidence interval, HER2 human epidermal growth factor receptor 2, HER3 human epidermal growth factor receptor 3, HR hazard ratio, H score histologic score, IHC immunohistochemistry, ISH in situ hybridization, ITT intention to treat, OS overall survival, PTEN phosphatase and tensin homolog. A OS by baseline characteristics and stratification factors. B OS by biomarker subgroups
Safety
Safety is summarized in Table 2. Incidences of any-grade AEs, fatal AEs, AEs that led to dose modifications, and cardiac AEs were similar between treatment arms. Incidence of symptomatic and asymptomatic left ventricular systolic dysfunction was low. Serious AEs, grade 3–5 AEs, and any-grade diarrhea were more frequent in the pertuzumab arm. Most diarrhea events were grade 1 or 2.
Discussion
Although JACOB failed to meet its primary endpoint [8], these end-of-study analyses confirm some evidence of activity. Median OS was 18.1 months in the pertuzumab arm and 14.2 months in the placebo arm at ≥ 44.4 months’ median follow-up (stratified HR 0.85 [95% CI, 0.72–0.99]), signifying a 15% reduction in risk of death when adding pertuzumab to trastuzumab and chemotherapy in patients with previously untreated metastatic GC/GEJC. At the primary analysis, the OS HR was 0.84 (95% CI, 0.71–1.00; p = 0.057); there were 242 and 262 OS events in the pertuzumab and placebo arms, respectively. Compared with the primary analysis, there was an increase in number of OS events and a decrease in the CI size in this end-of study analysis, thus supporting that the end-of-study analysis has increased statistical power when compared with the primary analysis.
The overall safety profile was acceptable and comparable between arms; however, the incidence of serious AEs, grade 3–5 AEs, and any-grade diarrhea was higher in the pertuzumab arm than in the placebo arm. There was no increase in the number of patients who experienced any-grade AEs; however, there was a slight increase in the number of patients who experienced serious AEs (final analysis: 45% and 39%; end-of-study analysis: 46.2% and 40.2%) and grade 3–5 AEs (final analysis: 80% and 73%; end-of-study analysis: 80.5% and 74.2%) in both the pertuzumab and placebo arms, respectively. Results from OS baseline characteristic and stratification factor subgroup analyses were consistent with those of the primary analysis [8]. Overall, biomarker analyses did not reveal a clear and consistent predictive effect for any of the markers tested. Consistent with biomarker results from GATSBY [10], increased OS was observed in both arms in patients with elevated or more homogeneously expressed HER2 levels (although this was not tested statistically). This suggests that gastric tumors with high and uniform HER2 expression may be more driven by HER2 signaling, and are more sensitive to anti-HER2 therapy. PTEN protein expression levels did not seem to affect OS. Total loss of PTEN protein, which is hypothesized to contribute to resistance to HER2-targeted therapy, could not be evaluated due to small sample size. The study design included HER2-targeted therapy in the control arm, which may limit detection of biomarker signals in this analysis. Therefore, only markers that produced large effects may have been detectable, while smaller effects may have been masked.
The limited treatment effect of combining pertuzumab with trastuzumab and chemotherapy in GC may be multi-factorial. Underlying differences in HER2 expression between GC and BC and the increased complexity of GC suggests that HER2 signaling may not be the only driver of disease progression in some patients [7]. Lapatinib, an anti-HER2 tyrosine kinase inhibitor, also failed to show improved OS as a first-line treatment in combination with capecitabine and oxaliplatin in patients with HER2-positive metastatic GC, esophageal, or gastroesophageal adenocarcinoma [11]. Similarly, trials of anti-HER2 therapies (trastuzumab, lapatinib, and ado-trastuzumab emtansine) in patients with previously treated HER2-positive metastatic GC have also failed to show improved OS [12,13,14]. Therefore, success in advancing treatment for HER2-positive metastatic GC has been limited to the advent of trastuzumab in combination with chemotherapy, which has remained the first-line standard of care for a decade. Recently, the U.S. Food and Drug Administration (FDA) granted accelerated approval for the use of pembrolizumab in combination with first-line trastuzumab and chemotherapy for the treatment of patients with HER2-positive metastatic GC [15], based on the interim data from the ongoing KEYNOTE-811 study (NCT03615326) [16]. In this study, pembrolizumab, trastuzumab, and chemotherapy showed significantly improved ORR vs. placebo, trastuzumab, and chemotherapy (74% vs. 52%) and a manageable toxicity profile, with similar incidence of AEs across arms; the median DoR was 10.6 months in the pembrolizumab arm and 9.5 months in the placebo arm [16]. In previously treated patients, trastuzumab deruxtecan demonstrated superior efficacy (ORR and OS) vs. physician’s choice of either irinotecan or paclitaxel monotherapy, becoming a new standard of care in the US [17]. There remains an unmet medical need in HER2-positive GC/GEJC, and a wide array of novel therapies and treatment regimens are currently under investigation in these patients; these include anti-HER2 monoclonal antibodies (margetuximab), checkpoint inhibitors (nivolumab and ipilimumab), tyrosine kinase inhibitors (tucatinib), and bispecific antibodies that target HER2 (zanidatamab) [18,19,20,21,22]. There have also been several developments in screening techniques, such as next-generation sequencing and liquid biopsy, which can supplement the traditional diagnostic tests to further understand the complexity of HER2-positive GC and identify targetable genomic alterations [23, 24]. In addition, multiple screening techniques (composite testing) or multiple samples taken from different sites or lesions can be used to assess HER2 heterogeneity within tumors, which can cause discordant results regarding the HER2 status between biopsies and could be associated with disease progression, to provide an accurate assessment of a patient’s HER2 status and allow selection of an optimal treatment regimen [25].
Limitations of the study include the lack of power for some of the subgroup analyses, and the resulting large, overlapping CIs.
Conclusions
Although JACOB did not meet its primary endpoint, the study continues to demonstrate some, albeit limited, evidence of treatment activity and an acceptable safety profile for pertuzumab plus trastuzumab and chemotherapy in previously untreated HER2-positive metastatic GC/GEJC after long-term follow-up (≥ 44.4 months).
Data availability
Qualified researchers may request access to individual patient-level data through the clinical study data request platform: https://vivli.org/. Further details on Roche's criteria for eligible studies are available here: https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
References
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19.
Swain S, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34.
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Vaile G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–31.
Nakamura Y, Okamoto W, Kato T, Esaki T, Kato K, Komatsu Y, et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med. 2021;27:1899–903.
Chugai Pharmaceuticals Co. Ltd. Chugai obtains regulatory approval for perjeta and herceptin for additional indication of HER2-positive colorectal cancer. 2022. https://www.chugaipharm.co.jp/english/news/detail/20220328160000_906.html. Accessed July 2022.
Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Panault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–50.
Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–84.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22.
Shah MA, Kang YK, Thuss-Patience PC, Ohtsu A, Ajani JA, Cutsem EV, et al. Biomarker analysis of the GATSBY study of trastuzumab emtansine versus a taxane in previously treated HER2-positive advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2019;22:803–16.
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2—positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—a randomized Phase III trial. J Clin Oncol. 2016;34:443–51.
Makiyama A, Sagara K, Kawada J, Kashiwada T, Hosokawa A, Horie Y, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT). J Clin Oncol. 2018;36:4011–4011.
Satoh T, Xu R-H, Chung HC, Sun G-P, Doi T, Xu J-M, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol. 2014;32:2039–49.
Thuss-Patience PC, Shah MA, Ohtsu A, Cutsem EV, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640–53.
US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for HER2-positive gastric cancer. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-her2-positive-gastric-cancer. Accessed 26 April 2022.
Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727–30.
Shitara K, Bang Y-J, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–30.
Catenacci DV, Rosales M, Chung HC, Yoon HH, Shen L, Moehler M, et al. MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021;17:1155–64.
Park H, Bekaii-Saab TS, Kim SS, Kamath SD, Pishvaian MJ, Chen C, et al. Phase 1b/2, open-label, dose-escalation and expansion trial of tucatinib in combination with trastuzumab with and without oxaliplatin-based chemotherapy or pembrolizumab in patients with unresectable or metastatic HER2+ gastrointestinal cancers. J Clin Oncol. 2022;40:TPS376–TPS376.
Meric-Bernstam F, Hamilton EP, Beeram M, Hanna DL, El-Khoueiry AB, Kang Y-K, et al. Zanidatamab (ZW25) in HER2-expressing gastroesophageal adenocarcinoma (GEA): results from a phase I study. J Clin Oncol. 2021;39:164–164.
Kang YK, Chen LT, Ryu MH, Oh DY, Oh C, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–47.
Tintelnot J, Goekkurt E, Binder M, Thuss-Patience P, Lorenzen S, Knorrenschild JR, et al. Ipilimumab or FOLFOX with Nivolumab and Trastuzumab in previously untreated HER2-positive locally advanced or metastatic EsophagoGastric Adenocarcinoma—the randomized phase 2 INTEGA trial (AIO STO 0217). BMC Cancer. 2020;20:503.
Connors D, Allen J, Alvarez JD, Boyle J, Cristofanilli M, Hiller C, et al. International liquid biopsy standardization alliance white paper. Crit Rev Oncol/Hematol. 2020;156:103112.
Díaz-Serrano A, Angulo B, Dominguez C, Pazo-Cid R, Salus A, Jiménez-Fonseca P, et al. Genomic profiling of HER2-positive gastric cancer: PI3K/Akt/mTOR pathway as predictor of outcomes in HER2-positive advanced gastric cancer treated with trastuzumab. Oncologist. 2018;23:1092–102.
Buckley NE, Forde C, McArt DG, Boyle DP, Mullan PB, James JA, et al. Quantification of HER2 heterogeneity in breast cancer—implications for identification of sub-dominant clones for personalised treatment. Sci Rep. 2016;6:23383.
Acknowledgements
We thank the patients, their families, and the investigators who participated in this study, and the central testing and biomarker laboratory (Targos Molecular Pathology GmbH, Kassel, Germany). Support for third-party writing assistance for this manuscript, furnished by Katie Wilson, Ph.D., of Health Interactions, was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Prior presentation
These analyses have been presented in part:
Tabernero J, Hoff PM, Shen L, et al. Pertuzumab (P) + trastuzumab (H) + chemotherapy (CT) for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (mGC/GEJC): Final analysis of a Phase III study (JACOB). Oral presentation at the ESMO 2017 Congress, Madrid, Spain; Sept 8–12, 2017; Abstract 2289.
Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomized, placebo-controlled phase III study. Lancet Oncol. 2018; 19(10):1372–1384.
Tabernero J, Hoff PM, Shen L, et al. End-of-study analysis from JACOB: A phase III study of pertuzumab (P) + trastuzumab (H) and chemotherapy (CT) in HER2-positive metastatic gastric or gastroesophageal junction cancer (mGC/GEJC). Oral presentation at the ESMO Virtual Congress 2020; Sept 19–21, 2020; Abstract 1423MO.
Funding
This study was sponsored by F. Hoffmann-La Roche Ltd. The sponsor, F. Hoffmann-La Roche Ltd, contributed to the design of this study. Data collected by the investigators were analyzed by statisticians at F. Hoffmann-La Roche Ltd. Authors employed by the study sponsor contributed to the conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, and approval of the manuscript, as well as the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
JT and HM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design were performed by JT, PMH, LS, AO, MAS, and Y-KK. Acquisition, analysis, or interpretation of data were performed by JT, PMH, LS, AO, MAS, AS, SH, HM, ER, AK, and Y-KK. Drafting of the manuscript was performed by JT. Critical revision of the manuscript for important intellectual content was performed by JT, PMH, LS, AO, MAS, AS, SH, HM, ER, AK, and Y-KK. Statistical analysis was performed by HM.
Corresponding author
Ethics declarations
Conflict of interest
All authors reported receiving research support in the form of third-party medical writing assistance for this manuscript, provided by F. Hoffmann-La Roche Ltd. Josep Tabernero reported receiving consulting fees from Array BioPharma, AstraZeneca, Avvinity Therapeutics, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech, Inc., HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati Therapeutics, Neophore, Novartis, Ona Biotechnology, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Scandion Oncology, Servier, Sotio Biotech, Taiho Pharmaceutical, Tessa Therapeutics, and TheraMyc (paid to self), and honoraria for educational events from Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education, and Physicians’ Education Resource (paid to self). Paulo M. Hoff reported receiving research grant/funding from F. Hoffmann-La Roche Ltd and is the President of the Brazilian Oncology Society (SBOC). Lin Shen reported receiving research grant/funding from Beihai Kangcheng Medical Technology, Baiji Shenzhou (Beijing) Biotechnology Co., Beijing Xiantong Biomedical Technology, Jacobio Pharmaceuticals, Qilu Pharmaceutical, Yaojie Ankang (Nanjing) Technology Co., and Zai Lab (paid to institution), consulting fees from Boehringer Ingelheim, Haichuang Pharmaceutical, Harbour Biomed, Merck, Mingji Biopharmaceutical, and MSD (paid to self), honoraria for speakers bureaus from CSTONE Pharmaceuticals, Jiangsu Hengrui Medicine, Hutchison Whampoa, and Zai Lab (paid to self), and has participated on advisory boards for AstraZeneca, BMS, CSTONE Pharmaceuticals, Daiichi Sankyo, F. Hoffmann-La Roche, Rongchang Pharmaceuticals, Sanofi, and Zai Lab. Atsushi Ohtsu reported receiving research grant/funding from F. Hoffmann-La Roche Ltd and BMS (paid to self), and honoraria for lectures from Chugai (paid to self). Manish A. Shah reported receiving research grants/funding from BMS, Merck, and Oncolys BioPharma (paid to institution). Asna Siddiqui and Sarah Heeson are employees of Roche Products Ltd and own shares in F. Hoffmann-La Roche Ltd. Astrid Kiermaier is an employee of and owns shares in F. Hoffmann-La Roche Ltd. Harrison Macharia is an employee of F. Hoffmann-La Roche Ltd. Eleonora Restuccia is an employee of and owns shares in F. Hoffmann-La Roche Ltd/Genentech Inc. Yoon-Koo Kang reported receiving consulting fees from ALX Oncology, Amgen, Blueprint, BMS, Daehwa Pharmaceutical, F. Hoffmann-La Roche, MacroGenics, MSD, Novartis, Surface Oncology, and Zymeworks (paid to self).
Human rights statement and informed consent
JACOB was performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation E6 guideline for Good Clinical Practice, or the laws and regulations of the country in which the research was conducted, whichever afforded greater protection to the individual. Approval for the protocol, any amendments, informed consent forms, information provided to patients, and relevant supporting information was obtained from institutional review boards and/or ethics committees at participating sites. All patients provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tabernero, J., Hoff, P.M., Shen, L. et al. Pertuzumab, trastuzumab, and chemotherapy in HER2-positive gastric/gastroesophageal junction cancer: end-of-study analysis of the JACOB phase III randomized clinical trial. Gastric Cancer 26, 123–131 (2023). https://doi.org/10.1007/s10120-022-01335-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-022-01335-4