Abstract
Background and aims
Endoscopic submucosal dissection (ESD) for undifferentiated early gastric cancer (UD EGC) has debate due to the risk of lymph node metastasis. We investigated the outcomes of ESD compared to those of surgery for the UD EGC within expanded indication.
Methods
We reviewed 971 UD EGC patients performed ESD across 18 hospitals in Korea and 1812 patients who underwent surgical resection in two hospitals between February 2005 and May 2015. Of these cases, we enrolled a curative resected ESD group of 328 patients and surgery group of 383 cases within an expanded indication. Overall outcomes and one-to-one propensity score-matched (218 ESD group vs 218 surgery group cases) outcomes for these two groups were analyzed.
Results
Over the 75.6 month median follow-up period for the 711 enrolled cases, recurrences occurred in 22 patients (6.7%) in the ESD group but not in the surgery group. Overall survival (OS) was higher in the surgery group (p = 0.0316) in all cases, but there was no significant difference after propensity score matching (p = 0.069). According to the histologic type in propensity score matching, the OS of signet ring cell carcinoma and poorly differentiated carcinoma patients did not differ between the ESD and surgery groups (p = 0.1189 and p = 0.3087, respectively). In the surgery group involving expanded criteria, lymph node metastasis was found in six cases (1.56%).
Conclusions
Although ESD shows comparable outcomes to surgery for the UD EGC within expanded indications, appropriate patient selection is needed for the ESD due to the possibility of lymph node metastasis.
Similar content being viewed by others
Introduction
In early gastric cancer (EGC) patients, endoscopic submucosal dissection (ESD) has produced favorable long-term outcomes and the indication criteria for this procedure are widening [1, 2]. The expanded indications for ESD consist of three discrete criteria that have been used for EGC: I, intramucosal tumor, differentiated type, without ulcerative findings, and > 2 cm in size; II, intramucosal tumor, differentiated type, with ulcerative findings, and ≤ 3 cm in size; and III, intramucosal tumor, undifferentiated type, without ulcerative findings, and ≤ 2 cm in size [3, 4]. However, there is still some debate regarding the appropriateness of using ESD for undifferentiated type EGC (UD EGC) due to the risk of lymph node metastasis.
Previous studies have investigated the long-term outcomes of ESD interventions for UD EGCs meeting the expanded indication criteria, which were found to be comparable to surgical treatments for this cancer [5,6,7,8,9,10]. Notably, however, there have been several case reports of lymph node or distant metastases arising after curative ESD in EGC patients meeting the expanded indications, and this treatment is, therefore, still under investigation with regard to these widened criteria [11,12,13,14]. It is notable also that in the expanded indication criteria, the evidence is still insufficient for undifferentiated-type tumors and superficially invasive submucosal tumors. In addition, some reports have indicated that lymph node metastases have occurred after surgery in some UD EGC patients when the tumor was included in the expanded indications [9, 15].
Most previous studies on the use of ESD to treat UD EGC included a relatively small number of cases from a single center, and also included non-curative resection cases which should be treated by surgery. To obtain greater clarity regarding the outcomes of ESD compared to surgery in the treatment of UD EGC, our current investigation was designed as a nationwide Korean study of only cases that underwent curative resection by ESD or surgery within the expanded indications.
Methods
Patients
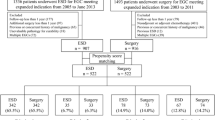
This was a multicenter, retrospective study of cases from 18 institutions across Korea. The study protocol was approved by the Institutional Review Board of the Hallym University Hospital, Korea (IRB number: 2017-08-002), and all participating centers also received committee approval. From February 2005 to May 2015, 971 patients at the 18 participating hospitals were diagnosed with UD EGC and underwent ESD. Initial multiple gastric cancers and previous history of gastric cancer were excluded. Of these cases, 643 patients were excluded from our further analyses, because their indications went beyond the expanded criteria. A total of 1812 patients received surgical resection for UD EGC in two hospitals during the same study period (National Cancer Center and Seoul National University Hospital). However, 1429 of these cases were excluded from the study cohorts as again the patient indications went beyond the expanded criteria. We finally enrolled 328 patients in the ESD group and 383 patients in the surgery group which were included in the expanded criteria (intramucosal tumor, undifferentiated type, without lymphovascular invasion, without ulcerative findings, and ≤ 2 cm in size) and retrospectively analyzed their clinical and demographic data. To provide a comparison of the oncologic outcomes between the treatment groups, 1:1 propensity score-matching was additionally performed and 218 patients in each group were analyzed (Fig. 1).
Survival status was collected from the medical records and claims data of the Korean National Health Insurance Corporation where disqualification of health insurance was regarded as mortality and maintenance of insurance on the date of screening (March 31, 2019) was considered as censoring.
Endoscopic procedure and surgery
All ESD procedures were performed by experienced gastrointestinal endoscopists using a single-channel endoscope (GIF-H260, GIF-Q260, GIF-2TQ260M, GIF-Q260J, or GIF-H180; Olympus Optical, Tokyo, Japan). In brief, the patients receiving the ESD were sedated and their cardiorespiratory functions were monitored throughout the procedure. The typical ESD procedure sequence involved marking, mucosal incision, and submucosal dissection with simultaneous hemostasis. After completion of the endoscopic resection, all non-bleeding visible vessels were coagulated.
In the enrolled study patients who underwent surgical resection, D1 or D1 + lymph node dissection was performed for cT1N0 tumors in accordance with the Japanese Gastric Cancer Association treatment guidelines [16]. A total, distal, or proximal gastrectomy was performed by experienced surgeons based on the location of the tumor.
Gross and histopathologic evaluation
Prior to the evaluations, the resected specimens were stretched, pinned to a polystyrene plate, and totally immersed in 10% neutral buffered formalin. After fixation, each specimen was grossly examined to identify the lesion and the closest resection margin. Specimens resected by ESD were sectioned serially at 2 mm-intervals parallel to an imaginary line drawn from the edge of the tumor to the closest resection margin. Surgically resected specimens were sectioned serially at 4–6 mm intervals. Each sliced tissue specimen was embedded in paraffin, and 5 μm sections were cut from each paraffin block and stained with hematoxylin and eosin. All samples were independently reviewed by each hospitals’ experienced gastrointestinal pathologists and those pathologic reports were reviewed in this study.
Follow-up schedule
Endoscopy follow-ups were initially performed at 2–3 months after ESD. Then, endoscopy with abdominal computed tomography (CT) were performed every 6–12 months for 3 years and annually thereafter at least for 5 years from the initial treatment. In the surgically resected patients, a follow-up endoscopy and abdominal CT was performed every 6 months for the first 2–3 years and then annually for 5 years.
Definitions
Macroscopic types of gastric carcinoma were classified in accordance with the Japanese classification system, i.e. type I (protruded), type IIa (superficial elevated), type IIb (flat), type IIc (superficial depressed), and type III (excavated) [17]. Types I and IIa were classified as elevated and types IIb, IIc, and III as flat/depressed.
Curative resection was defined as complete resection with a diameter ≤ 2 cm and a tumor confined to the mucosa, with no lateral or deep resection margin, no ulceration, or lympho-vascular invasion. A resection was considered non-curative when the tumor did not fulfill these criteria.
Recurrences were categorized as synchronous, metachronous, local, or as a distant metastasis. A synchronous recurrence was defined as a newly discovered lesion, excluding the ESD scar, within 12 months from the date of the index ESD procedure. Metachronous lesions were defined as a newly discovered lesions, again excluding the ESD scar, after more than 12 months from the date of the index procedure. A local recurrence was defined as a recurrence at the ESD site or in the surgery group as a recurrence at the site of the anastomosis. Distant metastases was defined as a recurrence at a lymph node, in the peritoneum, or at other organs after treatment. Pathologically, only adenocarcinoma lesions were considered to be recurrences.
The primary end point of this current study was overall survival (OS), which was defined as the time from the start of treatment to death from any cause. The secondary end point was recurrence-free survival (RFS), which included any cases of synchronous recurrence, metachronous recurrence, distant metastasis, or local recurrence of the gastric cancer.
Statistical analysis
The distributions of the clinical characteristics of the current study patients were summarized as mean (standard deviation) or median (interquartile range [IQR]) for continuous variables, and frequency (percentage) for categorical variables. To compare differences in the distributions between the two treatment groups, a Pearson’s Chi-square test or Fisher’s exact test for categorical variables was applied, whereas a t test or Wilcoxon rank-sum test was used for continuous variables as appropriate. Propensity score matching was performed to balance the distribution of the clinical characteristics between the two treatment groups. Seven variables including age, sex, American Society of Anesthesiologists (ASA) physical status, location, lesion size, depth of invasion, and morphology were used to perform propensity score matching. Finally, well-matched 1:1 subsets were determined using nearest neighbor matching. Survival curves were plotted using the Kaplan–Meier method and the differences in survival between the two study groups were evaluated using the Log-rank test. We calculated 5 year survival rates with a two-sided 95% confidence interval (CI) using the Kaplan–Meier method. The Cox proportional hazard model was used to estimate the hazard ratio (HR) and two-sided 95% CI. All results were reported with two-sided p values and statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC), and R software, version 3.6.2 (R Project for Statistical Computing).
Results
Clinicopathological outcomes in the total patient cohort
The baseline characteristics of the 711 enrolled UD EGC patients are listed in Table 1. The mean age was significantly higher in the ESD group than the surgery group (58.4 ± 12.8 vs 50.9 ± 10.9, p < 0.001) and underlying diseases were also more prevalent in the ESD group. Endoscopic findings showed that elevated type and lower third tumor locations were more frequent in the ESD group than in the surgery group (21.3% vs 5.5%, p < 0.001 and 46.3% vs 31.3%, p = 0.0002, respectively; Table 1). Among the 672 study patients for which information on any familial history of gastric cancer was available, 98 patients (14.6%) had a positive history of this cancer but there was no difference in this regard between the ESD and surgery groups (11.3% vs 15.9%, p = 0.2559).
In the pathologic findings, poorly differentiated adenocarcinoma (PDA) was more common in the ESD group (53.7% vs 29.8%, p < 0.001) as was lamina propria invasion (69.2% vs 52.7%, p < 0.001; Table 2). The median procedure time in the ESD group was 2 min (IQR 5–226 min).
Oncologic outcomes in the total patient cohort
All 22 recurrences among the total patient cohort were among the ESD cases with none arising in the surgery group (Table 2). Metachronous recurrences were described in 12 cases (3.7%) with a 34.5 month median follow-up period (IQR 25–52.5 months). Synchronous recurrences were detected in four cases (1.2%) over a 5 month median follow-up (IQR 3.7–6.7 months). Local recurrences arose in four cases (1.2%) over 26 months of follow-up (IQR 13.5–40 months). Finally, there were two patients with a distant metastatic recurrence (0.6%) over a median follow-up of 75.5 months (IQR 75.2–75.7 months). As shown in Fig. 2, additional ESD and surgery was performed in 12 and seven of these recurrent cases, respectively. One patient with a metachronous recurrence was lost to follow-up. One recurrent patient with an index lesion involving a 16 mm muscularis mucosal invasion PDA developed a liver metastasis at 75 months after ESD and died 17 months after chemotherapy. The remaining patient among the 22 recurrent cases had an index lesion associated with a 15 mm lamina propria invasion PDA but developed a retroperitoneal lymphadenopathy with bone metastasis at 76 months after the ESD and died 1 month after chemotherapy.
The 5-year OS rates were 96.1 and 98.7% in the ESD and surgery groups, respectively, with significance (HR 2.18, 95% CI 1.05–4.53, p = 0.0316). As shown in Fig. 3, univariable analysis indicated that age was risk factor for death in all patients (HR 1.06, 95% CI 1.04–1.09, p < 0.001). The 5-year RFS outcomes in the ESD group, taking into account the cases of synchronous recurrence, metachronous recurrence, distant metastasis, or local recurrence of gastric cancer, was 93.2%.
Oncologic outcomes of propensity score-matched patients
Propensity score-matching of the entire study population yielded 218 matched pairs in the ESD and surgery groups. The baseline and clinical characteristics, and the oncologic outcomes of the propensity score-matched patients are listed in Tables 3 and 4. The 5-year OS rates were 95.6 and 98.2% in the ESD and surgery groups without significant difference (HR 2.36, 95% CI 0.91–6.10, p = 0.069; Fig. 4). In subgroup analysis of the signet ring cell carcinoma (SRC) and PDA patients by propensity score-matching within the surgery group, the OS rate also showed no significant difference compared to surgery (HR 2.75, 95% CI 0.73–10.37, p = 0.1189 and HR 2.01, 95% CI 0.51–7.90, p = 0.3087, respectively; Fig. 4). The 5-year RFS in the ESD group was 95.2%; in the subgroup analysis, that in the SRC group was 98.1% and in the PDA group was 91.1%.
Lymph node metastases in the surgery group under the expanded indication criteria
Among the 383 study cases in the surgery group under the expanded indications, lymph node metastases were evident in six cases (1.56%) with a median age of 61 years (IQR 41.5–61.75 months) and median tumor size of 17 mm (IQR 12.5–19.5 mm). These patients received chemotherapy after surgery and no recurrence or distant metastasis was detected over the subsequent 60 month median follow-up period (IQR 54–63.75 months; Table 5).
Discussion
Endoscopic resection is an established treatment approach for EGCs that meet standard guidelines and extended criteria, including demonstrating a low risk of lymph node metastasis [1, 18, 19]. Notably, however, there are still concerns among clinicians regarding the results of ESD for UD-EGC within the expanded criteria due to the risk of lymph node metastasis [9, 10, 15, 16, 20,21,22] and there have been few studies involving large number of these cases with a multi-center design. In our present study, we attempted to address this by analyzing the outcomes in 313 cases who underwent an ESD for the curative resection of a UD EGC within the expanded indications from 18 centers across Korea. We then compared these patients with a cohort of surgically treated cases with the same cancer. Our results revealed that the surgery group had a better OS outcome than that ESD group, but that there were no significant OS differences between these two groups after propensity score matching. In our subgroup analysis, the SRC and PDA groups also showed no significant OS differences compared to the surgery group. As expected, the recurrence rate was higher in the ESD group (6.7%) compared to the surgery group (0%) due to a higher risk of recurrence with a larger remaining stomach. Notably however, there were two cases of a fatal distant metastasis after ESD and six cases of lymph node metastasis in our present surgery group with the expanded indications. Care should be taken therefore in selecting UD EGC patients for an ESD treatment, even with the expanded criteria.
Previous studies have reported a 90–95% 5-year survival rate for UD EGC after ESD with no statistically significant differences from the surgical outcomes for this cancer, although a tendency toward a better OS was evident in surgery cases [8,9,10, 23]. One of these prior reports found that the OS differed according to the histologic type with PDA patients showing a poorer OS than SRC cases [10]. We found in our current study series that the OS was better in the surgery group among the total patient cohort, but no significant difference was observed after propensity score matching. The reason for this discrepancy is quite likely to be patient age, which was revealed as a risk factor for death and was higher in the ESD group before matching. It was notable, however, that a longer OS trend was evident in our current surgery group even after propensity score matching (HR 2.36, 95% CI 0.91–6.1, p = 0.0775). Previous reports have explained this trend by histologic type differences as PDA lesions are typically highly associated with vertical cut-end positivity compared to SRCs, which can lead to poorer survival [10, 24]. In our current data, subgroup analysis indicated no OS differences in group comparisons of SRC vs surgery and PDA vs surgery. However, the two cases of distant metastasis after ESD in our present series had a PDA histology. Further studies of oncologic outcome trends according to the histologic type of GC will assist pathologists to distinguish between SRC and PDA tumors more clearly.
GC recurrences after an ESD can arise in the remaining stomach, leading to a higher risk of these occurring in this treatment group compared to a surgically treated group. The overall recurrence rate after ESD has been reported to be as high as 10% in some previous studies and can be reduced incomplete resected cases to around 4% [9, 10, 25, 26]. Metachronous recurrences in UD EGC patients are more frequent than synchronous or local recurrences [9, 10, 27] and can thus be more readily treated by an additional ESD procedure because the chance of another recurrence at the ESD scar will be decreased. In our present study, the total recurrence rate was 6.7% among the curative resected ESD cases and metachronous recurrences represented more than half of these (3.7%). Theoretically, local recurrent can not be happened in case with curative R0 resection, however, it often happened in clinical situation. We think that this local recurrence can be happened, because the recurred tumor is located very near the ESD site coincidently. Unlike recurrences of this nature, a distant metastatic recurrence is very difficult to cure and will typically lead to a fatal outcome. Our present results revealed that two of our current UD EGC patients developed a distant metastasis after curative ESD within the expanded indications but that both died after chemotherapy. We thus recommend close monitoring through regular endoscopy and CT scans, particularly in UD EGC cases. The frequency and duration of the surveillance after an ESD for UD EGC must also be considered carefully because most of the previously reported distant metastatic cases including those in our current series occurred within 5 years after this procedure [10].
Although a CT scan can support the pre-procedural diagnosis with regard to lymph node metastasis, the detection rate for this is still low. To address this problem, many researchers have reviewed surgical data retrospectively and have thereby generated indication criteria for ESD that included very low or negative risk of lymph node metastasis. Previous studies have also reported a rate of lymph node metastasis below 1% for UD EGC within the expanded indication criteria in surgical cases and recommended ESD for lesions of less than 1 cm in size [9, 28,29,30,31]. In our current study, however, we observed a 1.56% rate of lymph node metastasis in our expanded indication UD EGC series, and the smallest of these was a 4 mm PDA with lamina propria invasion. The possibility of a lymph node metastasis should, therefore, be determined before making any treatment decisions in UD EGC patients, even with the expanded indication criteria.
Although our current investigation was a multicenter, nationwide study that included a relatively large number of UD EGC patients that received a curative treatment within expanded indications by ESD or surgery, there were some notable limitations of our study design. There was an inevitable bias because of the retrospective nature of the analysis. However, we tried to reduce the extent of any selection bias using propensity score matching. A second limitation was that we could not unify the pathologic findings for the resected specimens, leading to possible discrepancies in the diagnosis of SRC and PDA, and existence of ulcer or scar. In addition, each of the participating hospital likely has have standards and protocols, and further pathological studies will be required to assess survival outcomes in accordance with a more precise ratio between SRC and PDA lesions. A third limitation was that we could not determine the exact cause of death due to the retrospective design of the study, and it was thus not possible to compare disease-specific survival rates. A fourth is that not all hospital perform immunohistochemical study for lymphovascular invasion and surgical specimen were sectioned serially at 4–6 mm intervals in Korea. Previous study showed that 2 mm wide section interval was suitable for the pathological evaluation of sm invasion or LVI [32]. Therefore, chance of missing and downsized estimation of lymphovascular invasion can be possible in this retrospective designed study. Finally, due to various circumstances, we only used surgical data from two of the participating hospitals as they could provide well-structured patient data. Despite these limitations, however, a principal advantage of our current report is that we analyzed a large number of t curative resection ESD cases within expanded indications compared to surgery cases.
In conclusion, although the outcomes of ESD are not inferior to surgery for the curative resection of UD EGC within expanded indications, the possibility of a lymph node metastasis after ESD remains. The decision to perform ESD in these patients should therefore be made carefully and this procedure should always be followed by regular closed monitoring with endoscopy and CT scans.
References
Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. GastrointestEndosc. 2011;74:485–93.
Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. GastrointestEndosc. 2015;81:333-341.e1.
Yao K, Uedo N, Kamada T, Hirasawa T, Nagahama T, Yoshinaga S, et al. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc. 2020a;32:663–98.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2020. https://doi.org/10.1007/s10120-020-01042-y
Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim DH, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. GastrointestEndosc. 2011;73:942–8.
Fukunaga S, Nagami Y, Shiba M, Ominami M, Tanigawa T, Yamagami H, et al. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. GastrointestEndosc. 2017;85:143–52.
Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, et al. Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: a non-inferiority-matched cohort study. Am J Gastroenterol. 2016;111:240–9.
Lee S, Choi KD, Han M, Na HK, Ahn JY, Jung KW, et al. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer. 2018;21:490–9.
Lim JH, Kim J, Kim SG, Chung H. Long-term clinical outcomes of endoscopic vs. surgical resection for early gastric cancer with undifferentiated histology. SurgEndosc. 2019;33:3589–99.
Park JC, Lee YK, Kim SY, Roh Y, Hahn KY, Shin SK, et al. Long-term outcomes of endoscopic submucosal dissection in comparison to surgery in undifferentiated-type intramucosal gastric cancer using propensity score analysis. SurgEndosc. 2018;32:2046–57.
Abe S, Oda I, Nakajima T, Suzuki H, Nonaka S, Yoshinaga S, et al. A case of local recurrence and distant metastasis following curative endoscopic submucosal dissection of early gastric cancer. Gastric Cancer. 2015;18:188–92.
Fujii H, Ishii E, Tochitani S, Nakaji S, Hirata N, Kusanagi H, et al. Lymph node metastasis after endoscopic submucosal dissection of a differentiated gastric cancer confined to the mucosa with an ulcer smaller than 30 mm. Dig Endosc. 2015;27:159–61.
Lee SH, Jee SR, Kim JH, Seol SY. Intramucosal gastric cancer: the rate of lymph node metastasis in signet ring cell carcinoma is as low as that in well-differentiated adenocarcinoma. Eur J GastroenterolHepatol. 2015;27:170–4.
Min BH, Kim ER, Kim KM, Park CK, Lee JH, Rhee PL, et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2015;47:784–93.
Lee JH, Choi IJ, Kook MC, Nam BH, Kim YW, Ryu KW. Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg. 2010;97:732–6.
Yao K, Uedo N, Kamada T, Hirasawa T, Nagahama T, Yoshinaga S, et al. (JGES Guidelines) Guidelines for Endoscopic Diagnosis of Early Gastric Cancer. Dig Endosc. 2020b. https://doi.org/10.1111/den.13684.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–25.
Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–8.
Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, et al. Risk factors for lymph node metastasis in undifferentiated early gastric cancer. Ann Surg Oncol. 2008;15:764–9.
Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–52.
Lee T, Tanaka H, Ohira M, Okita Y, Yoshii M, Sakurai K, et al. Clinical impact of the extent of lymph node micrometastasis in undifferentiated-type early gastric cancer. Oncology. 2014;86:244–52.
Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122–7.
Kim JH, Lee YC, Kim H, Song KH, Lee SK, Cheon JH, et al. Endoscopic resection for undifferentiated early gastric cancer. GastrointestEndosc. 2009;69:e1-9.
Ahn JY, Park HJ, Park YS, Lee JH, Choi KS, Jeong KW, et al. Endoscopic resection for undifferentiated-type early gastric cancer: immediate endoscopic outcomes and long-term survivals. Dig Dis Sci. 2016;61:1158–64.
Bang CS, Park JM, Baik GH, Park JJ, Joo MK, Jang JY, et al. Therapeutic outcomes of endoscopic resection of early gastric cancer with undifferentiated-type histology: a Korean ESD registry database analysis. ClinEndosc. 2017;50:569–77.
Lim JH, Kim SG, Choi J, Im JP, Kim JS, Jung HC. Risk factors for synchronous or metachronous tumor development after endoscopic resection of gastric neoplasms. Gastric Cancer. 2015;18:817–23.
Abe N, Watanabe T, Sugiyama M, Yanagida O, Masaki T, Mori T, et al. Endoscopic treatment or surgery for undifferentiated early gastric cancer? Am J Surg. 2004;188:181–4.
Park YD, Chung YJ, Chung HY, Yu W, Bae HI, Jeon SW, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7–10.
Kim HM, Pak KH, Chung MJ, Cho JH, Hyung WJ, Noh SH, et al. Early gastric cancer of signet ring cell carcinoma is more amenable to endoscopic treatment than is early gastric cancer of poorly differentiated tubular adenocarcinoma in select tumor conditions. SurgEndosc. 2011;25:3087–93.
Kwak DS, Min YW, Lee JH, Kang SH, Jang SH, Lee H, et al. Outcomes of endoscopic submucosal dissection for early gastric cancer with undifferentiated-type histology: a clinical simulation using a non-selected surgical cohort. Gut Liver. 2018;12:263–70.
Kim YI, Kook MC, Choi JE, Lee JY, Kim CG, Eom BW, et al. Evaluation of submucosal or lymphovascular invasion detection rates in early gastric cancer based on pathology section interval. J Gastric Cancer. 2020;20:165–75.
Acknowledgements
This work was supported by the Korean College of Helicobacter and Upper Gastrointestinal Research Foundation Grant (2017-08).
Funding
This study was funded by the Korean College of Helicobacter and Upper Gastrointestinal Research Foundation Grant (2017-08).
Author information
Authors and Affiliations
Contributions
JYA, WGS, and JC designed the study. JYA wrote the draft. JYA and YK analyzed the data. HY, SYN, BM, JJ, JHL, JK, WSL, BEL, MKJ, JMP, HLL, TG, MIP, JC, CHT, and YK collected and reviewed the data. BP performed statistical analysis. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author Ji Yong Ahn declares that he has no conflict of interest. Author Young-II Kim declares that he has no conflict of interest. Author Woon Geon Shin declares that he has no conflict of interest. Author Hyo-Joon Yang declares that he has no conflict of interest. Author Su Youn Nam declares that she has no conflict of interest. Author Byung-Hoon Min declares that he has no conflict of interest. Author Jae-Young Jang declares that he has no conflict of interest. Author Joo Hyun Lim declares that he has no conflict of interest. Author Jie-Hyun Kim declares that she has no conflict of interest. Author Wan Sik Lee declares that he has no conflict of interest. Author Bong Eun Lee declares that she has no conflict of interest. Author Moon Kyung Joo declares that he has no conflict of interest. Author Jae Myung Park declares that he has no conflict of interest. Author Hang Lak Lee declares that he has no conflict of interest. Author Tae-Geun Gweon declares that he has no conflict of interest. Author Moo In Park declares that he has no conflict of interest. Author Jeongmin Choi declares that he has no conflict of interest. Author Chung Hyun Tae declares that she has no conflict of interest. Author Young-Woo Kim declares that he has no conflict of interest. Author Boram Park declares that she has no conflict of interest. Author II Ju Choi declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not obtained from all individual participants included in the study due to retrospective designed study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahn, J.Y., Kim, YI., Shin, W.G. et al. Comparison between endoscopic submucosal resection and surgery for the curative resection of undifferentiated-type early gastric cancer within expanded indications: a nationwide multi-center study. Gastric Cancer 24, 731–743 (2021). https://doi.org/10.1007/s10120-020-01140-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-020-01140-x