Abstract
Background
The aim of this study is to compare surgical outcomes including postoperative complications and prognosis between total gastrectomy (TG) and proximal gastrectomy (PG) for proximal gastric cancer (GC). Propensity-score-matching analysis was performed to overcome patient selection bias between the two surgical techniques.
Methods
Among 457 patients who were diagnosed with GC between January 1990 and December 2010 from four Italian institutions, 91 underwent PG and 366 underwent TG. Clinicopathologic features, postoperative complications, and survivals were reviewed and compared between these two groups retrospectively.
Results
After propensity-score matching had been done, 150 patients (75 TG patients, 75 PG patients) were included in the analysis. The PG group had smaller tumors, shorter resection margins, and smaller numbers of retrieved lymph nodes than the TG group. N stages and 5-year survival rates were similar after TG and PG. Postoperative complication rates after PG and TG were 25.3 and 28%, respectively, (P = 0.084). Rates of reflux esophagitis and anastomotic stricture were 12 and 6.6% after PG and 2.6 and 1.3% after TG, respectively (P < 0.001 and P = 0.002). 5-year overall survival for PG and TG group was 56.7 and 46.5%, respectively (P = 0.07). Survival rates according to the tumor stage were not different between the groups. Multivariate analysis showed that type of resection was not an independent prognostic factor.
Conclusion
Although PG for upper third GC showed good results in terms of survival, it is associated with an increased mortality rate and a higher risk of reflux esophagitis and anastomotic stricture.
Similar content being viewed by others
Introduction
Gastric cancer (GC) remains a world-wide cancer with a high mortality rate [1]. In North American and some European countries, carcinoma of the cardia is the primary cancer type of GC, while there has been a tendency of incidence transmitting from distal toward proximal GC in Asia in recent years [2]. Cancer-related death incidence of proximal GC is higher than that of other sites of cancer of the stomach [3]. Proximal gastric cancer refers to cancers locating in gastric cardia and the upper third of stomach.
There are two different stomach resection types for proximal GC by surgical treatment: total gastrectomy (TG) and proximal gastrectomy (PG) [4].
Usually, the decision of gastro-intestinal surgeons depends on tumor size, tumor stage and volume of the remnant stomach. As common knowledge, as demonstrated in an elderly study [5] TG can achieve a longer tumor-free distal resection margin and more radical lymphadenectomy, which seems to have a better curative effect. The newly published “Japanese Gastric Cancer Treatment Guidelines 2010” recommends that PG is only suitable for some early stage diseases [6]. However, in most retrospective studies, TG had hardly showed superior results in this poor-outcome cancer compared to PG [7,8,9,10]. Also, anemia and weight loss are frequent postoperative complications in TG patients, which have to be considered [11]. Thus, the optimal extent of stomach resection for proximal GC is still controversial [4].
Some authors have reported that PG for early GC in the upper third of the stomach is an appropriate operation in terms of its radicality and safety [12,13,14] and support the notion that PG achieves survival rates equivalent to those of TG while preserving the physiologic functions of the gastric remnant [15].
In this multicenter western study, surgical results, such as postoperative complications and survival, were compared in patients who underwent TG or PG.
Methods
In this observational multicenter study, data were collected from the medical records of 457 patients who underwent resection with curative intent for histologically confirmed carcinoma of the upper third of the stomach from January 1990 through December 2010. Patients were operated on at four Italian centers experienced in gastric cancer treatment: Digestive Surgery, Catholic University of Rome (n = 102); 1st Division of General Surgery, University of Verona (n = 145); Department of Surgical Sciences, University of Insubria (Varese-Como) (n = 29); Department of Surgery, Vita-Salute San Raffaele University (Milan) (n = 181).
Among them, 91 patients (19.9%) underwent PG and 366 (80.1%) underwent TG.
Institutional Review Board approval has been preliminarily obtained in each center for the research purpose use of the data, stemming out from standard clinical practice, since no additional interventions were planned (multicenter observational study).
Upper third GC was defined as adenocarcinoma of the upper one third of the stomach with or without involvement of the esophagogastric junction, according to the classification of Japanese Gastric Cancer Association (JGCA) [6]. The location of the primary cancer was identified by esophagogastroscopy.
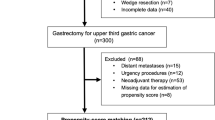
Patients with distant metastases (e.g., hepatic, lung, peritoneal dissemination or extraregional lymph nodes—superior mesenteric artery, middle colic artery, and para-aortic lymph nodes), those with less than 15 lymph nodes dissected, previous neoplastic diseases or a remnant GC, hematological pathologies, urgency procedures and those undergoing neoadjuvant treatments or who required a transthoracic esophagectomy were excluded from the study.
The extent of gastric resection depended on the judgement of the attending surgeon. In addition, tumor location and intraoperative verification of tumor-free resection margins were used to select patients for TG or PG.
For PG (including D1 + β lymph node dissection), the operative procedures included resection of the upper two-thirds of the stomach and the distal esophagus, followed by esophagogastrostomy with a 25-mm circular stapler. Gastric tube reconstruction was performed broadly as previously described [16].
After TG with D2 lymph node dissection, esophagojejunostomy (using a circular stapler, diameter 25 mm) was used routinely for Roux-en-Y reconstruction.
The residual tumor classification (R) and pathological staging were performed according to the UICC classification [17].
Clinical features, the variables of each type of operation (e.g., sex, age, tumor size, histological type, length of resection margin, numbers of retrieved, and metastatic lymph nodes), postoperative complications, and survivals were analyzed based on information obtained from medical records.
The follow-up was closed on December 2013; the median length of follow up was 43 months (range 1–152 months). At the time of the last follow-up, 215 patients (52%) were still alive, 26 (5.7%) were lost to follow-up and 215 (47%) had died from recurrence or other causes.
Postoperative morbidity was defined as a severity of grade 2 or more according to the Clavien–Dindo classification [18, 19]. Reflux esophagitis and anastomotic stricture were confirmed by endoscopic examination and biopsy along with associate symptoms, such as heart burn, regurgitation, and dysphagia.
To compare the baseline characteristics and clinicopathologic features between PG group and TG group, the χ2 test or Fisher’s exact test was used for categorical variables and the Student t test or the Mann–Whitney U test was used for continuous variables.
Propensity-score-matched analysis was conducted to reduce the effect of possible confounding factors and treatment-related selection bias [20]. Propensity scores were determined by a logistic regression model of the covariates. Using these propensity scores, patients in the PG group were individually matched to patients in the TG group. To assess bias reduction, we checked the balance of the matched data in terms of absolute standardized differences of covariates before and after matching. An absolute standardized difference of less than 10% suggests a substantial balance across the groups. The Kaplan–Meier method was used to estimate the long-term survival outcomes, and the log-rank test was used to analyze the statistical differences between the treatment groups. The relative risk (RR) for the long-term outcomes was determined with a Cox proportional hazards model. Multivariate analysis was performed with a multiple regression analysis, using the Cox proportional hazards model. P < 0.05 was considered statistically significant. Statistical analysis was performed using commercially available software (SPSS® for Windows version 20.0; Chicago, IL, USA).
The study flow diagram is shown in Fig. 1.
Results
Table 1 summarizes the clinicopathological features of all patients and propensity-score-matched patients. Overall, no significant association was found between operation type and sex, tumor size or Lauren classification. However, age, resection margin, tumor stage, numbers of lymph nodes retrieved, splenectomy, and multivisceral resections were found to be significantly different in the two groups.
In detail, patients undergoing PG were significantly older respect to patients of TG group (P = 0.012).
There was a higher splenectomy rate in the TG group (53.8%) than in the PG group (12.1%), (P < 0.001); whereas positive resection margin rate was higher in the PG group (8.8%) than in the TG group (3%), (P = 0.031).
The mean number of lymph nodes retrieved in the surgical specimen was significantly higher for TG (37.29 ± 18.7 nodes) than for PG (20.52 ± 10.4; P < 0.001), as well as the number of histopathologically positive lymph nodes (9.2 ± 4.6 vs 3.2 ± 2.7 respectively, P = 0.003).
According to TNM stages, the majority of patients in TG group had more advanced GC (P = 0.009) and 12 vs 3.3% of PG group (P < 0.001) underwent a multivisceral resection.
In 75 pairs of matched patients, all characteristics were similar between the groups, and post-matching standardized differences for all covariates were less than 10%.
The overall postoperative 30-day mortality rate after TG (1.3%—1 patient) was significantly lower than after PG (5.3%—4 patients) (P = 0.04) (Table 2). The patient in the TG group died due to an acute myocardial infarction.
Four patients in the PG group died in the postoperative period: in two cases due to septic shock related to anastomotic leak, in one case due to respiratory failure and in one case due to massive bleeding.
The grade III or grade IV (overall) surgical morbidity rate was 18.6% (25.3%) in the PG group and 20% (26.2%) in the TG group, respectively, (P = 0.084).
The clinical course after surgery is shown in Table 2.
There were no differences in major postoperative complications, including anastomotic leakage, intra-abdominal abscess, pancreatic fistula, and other surgery-related complications between the two groups. However, the incidences of reflux esophagitis and anastomotic stricture were significantly more common in the PG group compared with the TG group.
Mortality rate was significantly higher in PG group respect to TG group (5.3 vs 1.3%; P = 0.04).
Five-year overall survival for PG and TG group was 56.7 and 46.5%, respectively (P = 0.07) (Fig. 2).
When survival was analyzed within early stages (stage I and II), there were no differences in the 5-year survival rates between the two groups (98.5% for TG and 97.2% for PG, respectively (P = 0.62).
Multivariate Cox regression analysis showed that tumor stage, lymph node metastasis, and residual tumor were independent prognostic factors in patients with upper third GC. The extent of tumor resection was not an independent prognostic factor (Table 3).
Discussion
Proximal gastrectomy was introduced to improve patient performance status by conserving half of the stomach, and, thus, it was widely believed that proximal gastrectomy reduces postoperative weight loss. In addition, PG in the upper third of the stomach was believed to be appropriate in terms of both its radicality and safety [12,13,14].
To the best of our knowledge, our series represents the largest western experience comparing postoperative complications and prognosis between TG and PG in patients with proximal GC, and the only propensity-score-matched analysis in the literature.
In the West, Harrison et al. [14], from Memorial Sloan Kettering Cancer Center, in their experience on 98 proximal GC patients concluded that the extent of resection for proximal GC does not affect long-term outcome [14].
As previously reported, in our series the extent of gastric resection was at discretion of the attending surgeon; this aspect may justify both the higher rate of older patients in PG group and more advanced GC in TG group. In older patients, a limited surgery was probably chosen due to their multiple comorbidities. On the other hand, the high rate of TG, in advanced GC, can be justified by the purpose to achieve a radical resection.
As far as the weight loss is concerned, two studies showed comparable weight loss at all the same time points between the two surgical procedures [13, 15]. Another study reported that TG preserved weight loss better at postoperative 3 and 4 years, while no significant differences between groups were found at other time points (postoperative 1 and 6 month, 1, 2 and 5 years) [21].
Proximal gastrectomy is a considerable resection procedure for early stage of proximal GC providing that a sufficient distal resection margin can be ensured. This has been generally accepted by most surgeons [22, 23]. However, with respect to advanced diseases, it still has not reached a consensus. In Japan, many surgeons recommend TG to be the standard procedure to achieve a more radical effect for locally advanced GC located in the upper third of stomach. TG procedure comprises a more radical resection that prevents residual disease at the gastric margin and allows removal of all the perigastric lymph nodes. But some surgeons think the two are comparable in radical effect, because many published studies, including ours, showed that there is no significant difference in survival rate between PG and TG [7,8,9,10, 14, 24,25,26].
Oncologically, PG and TG should be equivalent procedures, provided clear resection margins are achieved, as the chance of metastasis to distal gastric nodes is uncommon [27].
As previously reported, in our experience, rates of anastomotic stricture and reflux esophagitis were markedly higher in the PG group (6.6 and 12%, respectively) respect to TG group (1.3 and 2.6%, respectively); but much lower respect to other studies.
Kim et al. [28] reported rates of esophagogastric anastomosis-site stricture and reflux esophagitis of 46.5 and 48% after proximal gastrectomy, respectively, and Katsoulis et al. [29] reported that 45 and 100% of patients experienced reflux symptoms after total gastrectomy and proximal gastrectomy, respectively. Others concluded that reflux after proximal gastrectomy was worse than after total gastrectomy and suggested that proximal gastrectomy should be avoided in adenocarcinoma of the gastric cardia, except in early cancer [30].
In a recent metanalysis incorporating nearly 1100 patients, Wen et al. [4] reported that PG was associated with higher morbidity, including increased reflux esophagitis and anastomotic stenosis. Still another study found increased rates of severe esophagitis in the TG group [31].
In a recent paper, Yamashita et al. [32] proposed a side overlap esophagogastrostomy to prevent reflux after PG, with excellent results in patients undergoing this procedure.
To the best of our knowledge, there is only one randomized controlled trial, published by Yoo et al. [33] that compared Roux-en-Y TG to PG with an unconventional reconstruction procedure (jejunal U-pouch interposition). Operation time tended to be shorter in TG group, but there was no significant difference between the two surgical procedures. Volume of intraoperative blood loss was obviously more by TG than PG procedure. The number of harvested lymph nodes in TG group was significantly more than that in PG group. Neither early nor late postoperative complication rates was different between two groups, but after 12 postoperative months the post-gastrectomy syndrome rate of TG procedure was significantly higher [33].
In his metanalysis, Wen and coworkers [4] concluded that, based on current retrospective evidences, TG and PG had similar overall survival outcome for proximal gastric cancer, but TG showed lower recurrence rate. PG with gastroesophagostomy had higher incidence of reflux esophagitis and anastomotic stenosis.
Respect to the impact of surgery on quality of life (QoL), it is unclear whether patients derive any benefit from the remaining distal stomach with a PG as opposed to performing a TG. The operative decision may then revolve around QoL and functional differences.
While multiple studies have been conducted to attempt to answer this question, many of them are hampered by a lack of validated QoL data [12, 14, 34].
Takiguchi et al. [35] reviewed nearly 400 patients of which 193 underwent proximal gastrectomy. Overall, QoL after PG was similar to QoL after TG, although PG patients benefited from reduced dumping and less need for additional meals. Some would argue that the removal of the lower esophageal sphincter in the setting of an intact distal stomach would predispose to reflux. While this could lead to a reduced QoL, a recent study showed that, even though one third of patients with PG had endoscopic signs of esophagitis, only two patients reported symptoms [36]. Karanicolas and coworkers found that patients undergoing PG developed significantly more clinical reflux and nausea, as well as a diminished global QoL compared to those undergoing TG or distal gastrectomy [37].
The present study had several limitations. First, the evidence of a case-matched retrospective analysis is not as well established as that of a randomized controlled trial. Case matching using propensity-score analysis could not offset all biases. The case matching in the present study was performed with a matching ratio of 1:1 without regard to the considerably lower frequency of PG compared with TG. Overall, three quarters of the patients who underwent TG were excluded from the present analysis. In some of them, the tumor status was even similar to that of the patients in the PG group. The patient selection, which was not equivalent between the two groups, might have influenced the results. Second, the matched patients included in the present analysis were a subgroup of patients who exhibited some distinct clinical features. The results of the present study might not necessarily be applicable to all upper third GC patients. For example, early gastric cancer is not common in most of the world. Thus, PG might be applicable only in countries with a high incidence of gastric cancer.
Third, the lack of a long-term follow-up on nutritional status and the evaluation of long-term QoL of patients limit a lot the real benefit of a sparing-organ technique, such as PG.
In conclusion, this study was the first comparative case-matched analysis between PG and TG for upper third GC and the largest western experience.
The role of PG is still uncertain. While PG is an equivalent oncologic procedure to TG for early stage GC, it may predispose to worsened clinical reflux and QoL. There do not seem to be any obvious QoL benefits to PG, and patients seem to manage reasonably well without a remnant distal stomach.
Only future prospective randomized trials would clarify the real benefits of a surgical procedure with respect to another.
References
Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(8):S4–66.
Deans C, Yeo MS, Soe MY, et al. Cancer of the gastric cardia is rising in incidence in an Asian population and is associated with adverse outcome. World J Surg. 2011;35(3):617–24.
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354–62.
Wen L, Chen XZ, Wu B, et al. Total vs proximal gastrectomy for proximal gastric cancer: a systematic review and meta-analysis. Hepatogastroenterology. 2012;59:633–40.
Papachristou DN, Fortner JG. Adenocarcinoma of the gastric cardia. The choice of gastrectomy. Ann Surg. 1980;192(1):58–64.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112.
Jakl RJ, Miholic J, Koller R, et al. Prognostic factors in adenocarcinoma of the cardia. Am J Surg. 1995;169(3):316–9.
Erturk MS, Ciçek Y, Ersan Y, et al. Analysis of clinicopathological prognostic parameters in adenocarcinoma of the gastric cardia. Acta Chir Belg. 2003;103(6):611–5.
Smith JW, Brennan MF. Surgical treatment of gastric cancer. Proximal, mid and distal stomach. Surg Clin N Am. 1992;72(2):381–99.
Stipa S, Di Giorgio A, Ferri M. Surgical treatment of adenocarcinoma of the cardia. Surgery. 1992;111(4):386–93.
Carey S, Storey D, Biankin AV, et al. Long term nutritional status and quality of life following major upper gastrointestinal surgery—a cross-sectional study. Clin Nutr. 2011;30(6):774–9.
Katai H, Sano T, Fukagawa T, et al. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. 2003;90:850–3.
Shiraishi N, Adachi Y, Kitano S, et al. Clinical outcome of proximal versus total gastrectomy for proximal gastric cancer. World J Surg. 2002;26:1150–4.
Harrison LE, Karpeh MS, Brennan MF. Total gastrectomy is not necessary for proximal gastric cancer. Surgery. 1998;123(2):127–30.
An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg. 2008;196:587–91.
Cordiano C, Mangiante G, Giacopuzzi S, de Manzoni G. Proximal gastrectomy: technical notes. In: de Manzoni G, Roviello F, Siquini W, editors. Surgery in the multimodal management of gastric cancer. Springer-Verlag Italia; 2012. p. 247–250.
Sobin LH, Wittekind C, Gospodarowicz M, editors. TNM classification of malignant tumors (UICC). 7th ed. New York: Wiley; 2009. p. 73–7.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(187–96):14.
D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81.
Kondoh Y, Okamoto Y, Morita M, et al. Clinical outcome of proximal gastrectomy in patients with early gastric cancer in the upper third of the stomach. Tokai J Exp Clin Med. 2007;32(2):48–53.
Kaibara N, Nishimura O, Nishidoi H, et al. Proximal gastrectomy as the surgical procedure of choice for upper gastric carcinoma. J Surg Oncol. 1987;36(2):110–112.
Kitamura K, Yamaguchi T, Okamoto K, et al. Total gastrectomy for early gastric cancer. J Surg Oncol. 1995;60(2):83–8.
Kitamura K, Yamaguchi T, Nishida S. The operative indications for proximal gastrectomy in patients with gastric cancer in the upper third of the stomach. Surg Today. 1997;27(11):993–8.
Kobayashi T, Sugimura H, Kimura T. Total gastrectomy is not always necessary for advanced gastric cancer of the cardia. Dig Surg. 2002;19(1):15–21.
Yoo CH, Sohn BH, Han WK, et al. Long-term results of proximal and total gastrectomy for adenocarcinoma of the upper third of the stomach. Cancer Res Treat. 2004;36(1):50–5.
Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;210:596–602.
Kim JH, Park SS, Kim J, et al. Surgical outcomes for gastric cancer in the upper third of the stomach. World J Surg. 2006;30:1870–6.
Katsoulis IE, Robotis JF, Kouraklis G, Yannopoulos PA. What is the difference between proximal and total gastrectomy regarding postoperative bile reflux into the oesophagus? Dig Surg. 2006;23:325–30.
Hsu CP, Chen CY, Hsieh YH, et al. Esophageal reflux after total or proximal gastrectomy in patients with adenocarcinoma of the gastric cardia. Am J Gastroenterol. 1997;92:1347–50.
Son MW, Kim YJ, Jeong GA, Cho GS, Lee MS. Long-term outcomes of proximal gastrectomy versus total gastrectomy for upper-third gastric cancer. J Gastric Cancer. 2014;14:246–51.
Yamashita Y, Yamamoto A, Tamamori Y, Yoshii M, Nishiguchi Y. Side overlap esophagogastrostomy to prevent reflux after proximal gastrectomy. Gastric Cancer. 2017;20:728–35.
Yoo CH, Sohn BH, Han WK, et al. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: prospective randomized study. World J Surg. 2005;29(12):1592–9.
Khan O, Goh S, Byrne B, Somers S, Mercer S, Toh S. Long-term outcomes of extended proximal gastrectomy for oesophagogastric junctional tumours. World J Surg. 2011;35:2245–51.
Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, Ota M, Iwasaki Y, Uchida N, Kodera Y, Nakada K. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18:407–16.
Ronellenfitsch U, Najmeh S, Andalib A, Perera RM, Rousseau MC, Mulder DS, Ferri LE. Functional outcomes and quality of life after proximal gastrectomy with esophagogastrostomy using a narrow gastric conduit. Ann Surg Oncol. 2015;22:772–9.
Karanicolas PJ, Graham D, Gönen M, Strong VE, Brennan MF, Coit DG. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg. 2013;257:1039–46.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
For this type of study, formal consent is not required.
Human and animal rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Rights and permissions
About this article
Cite this article
Rosa, F., Quero, G., Fiorillo, C. et al. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer–GIRCG). Gastric Cancer 21, 845–852 (2018). https://doi.org/10.1007/s10120-018-0804-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-018-0804-3