Abstract
Background
Gastric schwannomas are not common but are clinically important in terms of differential diagnosis from other submucosal lesions. The precise preoperative diagnosis, however, has been challenging mainly owing to the lack of specific findings in conventional imaging studies. The aim of this study was to revisit the possibilities and limitations of modern preoperative diagnostic modalities for gastric schwannomas.
Methods
Fourteen consecutive patients with a final pathological diagnosis of gastric schwannoma were retrospectively analyzed. Data included demographics, preoperative imaging studies/diagnosis, surgery, histopathology, and follow-up results.
Results
The series included 6 males and 8 females, with a median age of 49 years (range 26–68 years). No symptoms were presented, except for 1 patient with epigastric pain. The tumors were located in the upper (n = 5), middle (3), and lower stomach (6), with a median size of 41 mm (range 20–75 mm). Twelve schwannomas (86 %) showed homogeneous enhancement on computed tomography. Ulceration was seen on endoscopy in 4 of 12 available cases (33 %). Positron emission tomography was performed in the last 4 patients, showing fluorodeoxy-glucose uptake in all cases (100 %). A preoperative diagnosis of schwannoma was not obtained in the majority of cases (13/14, 93 %); only 1 case was correctly diagnosed, by endoscopic aspiration cytology. Laparoscopic partial gastrectomy was attempted and completed in 13 cases. The patients have been followed up for 4.7 years (range 2.1–20.3 years), with no recurrencesor metastases and acceptable gastrointestinal function.
Conclusions
The precise preoperative diagnosis of gastric schwannomas remains difficult even with modern imaging studies. Surgery, therefore, should be positively considered for patients without a conclusive preoperative diagnosis.
Similar content being viewed by others
Introduction
Schwannomas are neurogenic tumors that consist of excessive proliferations of Schwann cells which normally wrap around the axons of peripheral nerves [1]. Schwannomas rarely occur in the digestive tract, but when they do the most common site is the stomach, and they represent 0.2 % of all gastric tumors [2, 3]. There are several case reports of gastric schwannomas; however, each involved only a small number of patients. The clinical, radiologic, and endoscopic features of gastric schwannomas have not been specific enough to enable precise preoperative diagnosis.
In this study, we retrospectively analyzed 14 consecutive cases of gastric schwannomas, and we report the possibilities and limitations of preoperative evaluation using modern imaging modalities such as image-enhanced endoscopy, high-resolution computed tomography (CT), and nuclear medicine imaging. To our knowledge, this is one of the largest published series of gastric schwannomas with detailed preoperative imaging characteristics as well as clinical and surgical findings.
Patients, materials, and methods
Fourteen consecutive patients with gastric schwannomas (treated between 1997 and 2009), diagnosed pathologically in their surgical specimens, were involved in this retrospective study. The institutional review board approved the study and did not require additional informed consents for reviewing the patients’ medical records and images. Data included: (1) patient demographics; (2) preoperative findings of endoscopy, CT, positron emission tomography (PET), and preoperative diagnosis; (3) surgery and its outcomes; (4) histopathology; and (5) follow-up results. Data are shown as medians (ranges).
Preoperative evaluation
Endoscopy
Esophagogastroduodenoscopy was routinely performed by expert endoscopists, using a flexible gastrointestinal endoscope (GIF-XQ260, GIF-Q260J, and/or GIF-H260Z; Olympus Medical Systems, Tokyo, Japan) with a local anesthetic spray. Endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) biopsy was selectively performed in the 2 most recent patients. The images were stored in the database and were incorporated into the medical charts with the examiner’s findings.
Computed tomography
Abdominal CT scans were routinely performed as preoperative work-up. In all patients, an intravenous contrast medium (Iomeron; Eisai Pharmaceutical, Tokyo, Japan) was used to enhance the images. The gastric lumen was dilated with a bloating agent (Baros effervescent granules; Horii Pharmaceutical, Tokyo, Japan) when necessary to further clarify the localization and growth pattern of the lesions. The most recent patients underwent multi-detector (MD) CT scanning (Aquilion ONE; Toshiba Medical Systems, Tokyo, Japan). The images were incorporated into the medical charts with board-certified radiologists’ findings.
Positron emission tomography
Positron emission tomography (PET) was performed in the last 4 consecutive patients, using a Gemini GXL device (Royal Philips Electronics, Tokyo, Japan). A maximal standardized uptake value (SUV max) of more than 2.0 was considered “positive” by radiologists. These images were reviewable on the electric medical chart systems.
Surgery
All patients underwent surgical treatment. Procedures, operative times, blood loss, morbidity, and length of hospital stay were prospectively registered in the dedicated surgical database and were retrospectively analyzed.
Histopathology
Specimens were obtained from all 14 patients. All tissues were fixed in 10 % buffered formalin and embedded in paraffin. All archival hematoxylin- and eosin-stained slides were examined for each case by board-certified pathologists. Immunohistochemical staining was performed for c-kit, CD34, and S-100 protein. Data were incorporated into the medical charts with conclusive pathological diagnoses.
Follow up
The patients were followed up on an outpatient basis annually for 5 years. Follow up included physical examination, blood counts, serum chemistries, CT, and endoscopy.
Results
Patients’ backgrounds
The studied population consisted of 6 males and 8 females, with ages that ranged from 26 to 68 years (median 49 years). Thirteen patients (93 %) were referred to our institution after routine medical check-ups. No clinical symptoms were presented at the initial referral, except for 1 patient with mild and episodic epigastric pain. Body weight loss was not seen in any of the patients.
Preoperative evaluation
Table 1 summarizes the results of preoperative evaluation using endoscopy, CT, and PET scans. Localization of the tumor was primarily determined by endoscopy. Evaluation of the tumor growth pattern, however, was not always possible by endoscopy, and therefore this was mainly done by gastric CT scan. The size of the tumor, again, was determined based on CT scan findings.
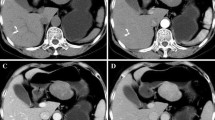
On endoscopy, the tumors were located in the upper (n = 5), middle (n = 3), and lower stomach (n = 6). Ulceration was seen in 4 of 12 available cases (33 %, Fig. 1a). Biopsy was attempted for 3 patients, but all 3 cases were diagnosed as normal mucosa. Endoscopic EUS-guided FNA biopsy was attempted for 2 patients, and we were able to make a preoperative diagnosis of gastric schwannoma in 1 of these patients. CT scans demonstrated the locations of each tumor, similar to the endoscopic findings, with a median size of 40 mm (range 20–75 mm). CT scans with contrast medium showed a homogeneous enhancement pattern in 12 of the 14 cases (86 %; Fig. 1b). The tumor growth patterns were diverse: intramural (n = 6), endoluminal (n = 4), and exogastric (n = 4). When performed, PET showed marked fluoro-deoxyglucose (FDG) uptake on the tumorous site in 4 out of 4 recent cases (100 %) with SUV max values that ranged from 3.3 to 6.8 (median 4.7) (Fig. 2).
Positron emission tomography (PET) findings. Fluorodeoxy-glucose (FDG)-PET shows high accumulation coincident with a gastric tumor. a Gastric schwannoma in a 62-year-old woman [standardized uptake value (SUV) max 5.9]. The arrow indicates FDG uptake at the tumor site. b Gastric schwannoma in a 61-year-old man [standardized uptake value (SUV) max 3.5]. The arrow indicates FDG uptake at the tumor site
The preoperative diagnoses obtained based on the above preoperative work-up data were: gastrointestinal stromal tumors (GISTs; n = 12), leiomyoma (n = 1), and gastric schwannoma (n = 1).
Surgery
All patients underwent partial gastrectomy (Figs. 3, 4; Table 2). Minimally invasive approaches, including standard laparoscopic surgery (n = 9), single-incision laparoscopic surgery (n = 2), and transvaginal natural orifice transluminal endoscopic surgery (NOTES; n = 2), were attempted in all patients, resulting in completion in 13 patients (93 %). One patient, with a preoperative diagnosis of GIST, was converted to open surgery owing to suspected tumor invasion into the surrounding tissue. Partial gastrectomy was still possible in this particular patient after meticulous dissection was performed under direct vision. The median operating time was 145 min (range 105–355 min), with median estimated blood loss of 100 ml (range 0–650 ml). There was neither major morbidity nor mortality in the series. The median hospital stay was 16 days (range 12–23 days).
Photographs showing operative findings of the tumor in the 62-year-old woman whose FDG-PET findings are shown in Fig. 2a. Transvaginal natural orifice transluminal endoscopic surgery (NOTES) partial gastrectomy was performed for the gastric schwannoma in this patient. a, b There is an extragastric 6.5-cm mass on the greater curvature of the stomach
Histopathology
The final diagnosis of gastric schwannoma was made by confirming the presence of spindle cells, which were positive for S-100 protein, and negative for CD34, c-kit, α-smooth muscle actin (SMA), and desmin (Fig. 5). In all cases, the spindle cells of schwannoma were positive for S-100 protein, and negative for CD34, c-kit, α-SMA, and desmin.
Follow up
Five-year follow up with annual endoscopy and/or CT was completed in 6 patients. The other 8 patients, including 5 patients with ongoing annual follow up, have shown no recurrences or metastases in 3 years (range 2.2–4.3 years). The function of the gastric remnant has been well maintained postoperatively. No gastrointestinal symptoms such as dyspepsia and bloating were observed in the series. Excessive body weight loss was not seen either.
Discussion
Schwannomas have a predilection for the head, neck, and flexures or surfaces of the limbs, but they can occur anywhere in the body [1]. Schwannomas of the gastrointestinal tract have rarely been reported and have occurred predominantly in the stomach [2]. It is reported that gastric schwannomas represent 0.2 % of all gastric tumors [3]. Gastric schwannomas arise from the nerve sheath of Auerbach’s plexus or, less commonly, Meissner’s plexus. These tumors are slowly growing encapsulated tumors composed of schwann cells in a collagenous matrix. As the tumor enlarges, it displaces the nerve to the periphery of the tumor, preserving neural function [4]. Gastric schwannomas occur more frequently in the fifth to sixth decade of life and more commonly in females [3, 5]. They are often asymptomatic and can be discovered incidentally at laparotomy or radiographically [6]. In their review, Bruneton et al. reported that, when symptomatic, most patients presented with bleeding, followed by abdominal pain [7].
As diagnostic modalities for gastric schwannomas, endoscopy, CT, and, recently, PET have been proposed. On endoscopy, gastric schwannomas appeared as elevated submucosal masses, and a central ulcer was seen in 25–50 % [2]. Endoscopic biopsies, when performed, can lead to false-negative results because of normal mucosa overlying a submucosal lesion [7]; therefore, endoscopic biopsy has been reported to be not as effective as expected in the diagnosis of gastric schwannomas [6]. EUS-FNA biopsy is currently considered the standard method for samples of submucosal tumors (SMTs). Mekky et al. [8] reported that EUS-guided FNA was an accurate method for the diagnosis of gastric SMTs, and the diagnostic yield was 43.3 %. Recently, de la Serna-Higuera et al. [9] reported that EUS-guided needle-knife incision and forceps biopsy (SINK biopsy) could be a reliable alternative to conventional FNA, providing larger samples that improve the histologic yield for upper gastrointestinal (GI) subepithelial tumors (SETs), but there are few reports about SINK biopsy and it is not yet an established technique. With the positive adoption of these modern sampling techniques, the preoperative differential diagnosis of gastric SMTs could be improved. On CT examination, gastric schwannoma displayed homogeneous enhancement in most cases and cystic change was uncommon [2]. Recently, FDG uptake on PET has been reported for schwannomas that originated from the extremities [10], retroperitoneum [11], esophagus [12], colon [13], and appendix [14].
However, detailed PET findings regarding schwannomas of gastric origin have been only sporadically reported; with 1 case reported by Komatsu et al. [15], and 2 cases reported by Ohno et al. [16]. Large accumulations of FDG in schwannomas may result in the potential ability of schwann cells to transport glucose for axonal repolarization [17].
Our present series, which involved 14 cases, is one of the largest published series of gastric schwannomas with detailed data of preoperative imaging studies. Demographically, there was a predominance of females, and 13 of 14 patients were asymptomatic with suspicions raised in routine medical check-ups. The endoscopic findings in these patients were comparable to those in previous reports in terms of location and mucosal change. Our CT findings, including high-resolution MDCT, were also comparable to previous reports in terms of enhancement patterns and growth patterns. To summarize our results, no additional information specific to gastric schwannomas was obtained even with the above modern imaging studies. As for surgery, minimally invasive approaches were successfully adopted, resulting in acceptable postoperative oncologic, functional, and cosmetic outcomes.
In conclusion, the precise preoperative diagnosis of gastric schwannomas remains difficult even with recent modern imaging modalities such as MDCT and PET. Because surgical resection can be performed safely with minimal invasiveness and acceptable postoperative GI function, surgery as “total biopsy” should be positively considered for patients with gastric SMTs with inconclusive preoperative imaging findings.
References
Enzinger FM, Weiss SW. Benign tumors of peripheral nerves. In: Enzinger FM, Weiss SW, editors. Soft tissue tumors. 2nd ed. St Louis: Mosby; 1988. p. 725–35.
Hong HS, Ha HK, Won HJ, Byun JH, Shin YM, Kim AY, et al. Gastric schwannomas: radiological features with endoscopic and pathological correlation. Clin Radiol. 2008;63:536–42.
Melvin WS, Wilkinson MG. Gastric schwannoma: clinical and pathologic conditions. Am Surg. 1993;59:293–6.
McNeer G, Pack GT. Neoplasms of the stomach. Philadelphia: J.B. Lippincott; 1974. p. 518–40.
Sarlomo-Rikala M, Miettinen M. Gastric schwannoma: a clinicopathological analysis of six cases. Histopathology. 1995;27:355–60.
Lin CS, Hsu HS, Tsai CH, Li WY, Huang MH. Gastric schwannoma. J Chin Med Assoc. 2004;67:583–6.
Bruneton JN, Drouillar J, Roux P, Ettore F, Lecomte P. Neurogenic tumors of the stomach: report of 18 cases and review of the literature. RoFo. 1983;139:192–8.
Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–9.
de la Serna-Higuera C, Pérez-Miranda M, Díez-Redondo P, Gil-Simón P, Herranz T, Pérez-Martín E, et al. EUS-guided single-incision needle-knife biopsy: description and results of a new method for tissue sampling of subepithelial GI tumors (with video). Gastrointest Endosc. 2011;74:672–6.
Ahmed AR, Watanabe H, Aoki J, Shinozaki T, Takagishi K. Schwannoma of the extremities: the role of PET in preoperative planning. Eur J Nucl Med. 2001;10:1541–51.
Hirai K, Umesaki N, Sumi T, Isiko O, Kanaoka Y, Ogita S, et al. Combined diagnostic imaging for retroperitoneal schwannoma. Oncol Rep. 2001;8:773–5.
Toyama E, Nagai Y, Baba Y, Yoshida N, Hayashi N, Miyanari N, et al. A case of thoracoscopically resected benign esophageal schwannoma with high uptake on FDG-PET. Esophagus. 2008;5:167–70.
Wang CL, Neville AM, Wong TZ, Hall AH, Paulson EK, Bentley RC. Colonic schwannoma visualized on FDG PET/CT. Clin Nucl Med. 2010;35:181–3.
Nishio M, Tamaki T, Hara M, Shibamoto Y. Appendiceal schwannoma detected by FDG-PET/CT. Clin Nucl Med. 2010;35:379–80.
Komatsu D, Koide N, Hiraga R, Furuya N, Akamatsu T, Uehara T, et al. Gastric schwannoma exhibiting increased fluorodeoxyglucose uptake. Gastric Cancer. 2009;12:225–8.
Ohno T, Ogata K, Kogure N, Ando H, Aihara R, Mochiki E, et al. Gastric schwannomas show an obviously increased fluorodeoxyglucose uptake in positron emission tomography: report of two cases. Surg Today. 2011;41:1133–7.
Beaulieu S, Rubin B, Djang D, Conrad E, Turcotte E, Eary JF. Positron emission tomography of schwannomas: emphasizing its potential in preoperative planning. AJR Am J Roentgenol. 2004;182:971–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujiwara, S., Nakajima, K., Nishida, T. et al. Gastric schwannomas revisited: has precise preoperative diagnosis become feasible?. Gastric Cancer 16, 318–323 (2013). https://doi.org/10.1007/s10120-012-0186-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-012-0186-x