Abstract
Background
In patients with stage II/III gastric cancer, tumors often recur even after curative D2 gastrectomy followed by adjuvant S-1 chemotherapy. The objective of this retrospective study was to clarify the prognostic factors in these patients that might be useful for future patients.
Methods
Overall survival (OS) was examined in 82 gastric cancer patients who underwent curative D2 surgery; were diagnosed with stage IIA, IIB, IIIA, IIIB, or IIIC pathologically; and received adjuvant S-1 after surgery between June 2002 and March 2010.
Results
When length of OS was evaluated by the log-rank test, significant differences were observed with regard to macroscopic tumor diameter and the depth of tumor invasion. A macroscopic tumor diameter >70 mm was regarded as a critical point of classification considering survival. Univariate and multivariate Cox’s proportional hazard analyses demonstrated that macroscopic tumor diameter was the only significant independent prognosticator. The 5-year survival was 64.9% in patients with a macroscopic tumor diameter <70 mm, and 33.1% in patients with a macroscopic tumor diameter ≥70 mm (P = 0.022).
Conclusions
The macroscopic tumor diameter was the most important prognostic factor for survival in patients with stage II/III gastric cancer who underwent D2 gastrectomy followed by adjuvant S-1 chemotherapy. Prognostic factors can be affected by adjuvant chemotherapy.
Similar content being viewed by others
Introduction
Every year, more than 934,000 people develop gastric cancer worldwide. Gastric cancer is the second most frequent cancer-related cause of death after lung cancer [1]. Complete resection is essential for the cure of gastric cancer. Stage IV cancers are unresectable, and these patients have a poor prognosis. Stage I cancers, in which the tumor is limited to T1N0–1 and T2N0, rarely develop a recurrence, and patients have an excellent prognosis. On the other hand, patients with stage II/III gastric cancer often develop tumor recurrence even after complete curative resection. Therefore, it is important to identify prognostic factors for patients with stage II and III gastric cancer in order to select patients for more aggressive treatment. Previously, lymph node metastasis [2, 3] and the depth of tumor invasion [4, 5] were reported to be significant prognostic factors that could be used to predict survival. However, these reports only analyzed patients who were treated with surgery alone or with surgery followed by adjuvant chemotherapy of unknown efficacy, because effective adjuvant chemotherapy had not been verified in these patients.
In 2007, the adjuvant chemotherapy trial of TS-1 for gastric cancer (ACTS-GC) trial demonstrated that S-1 was effective as adjuvant chemotherapy for Japanese patients who had undergone a D2 curative gastrectomy for locally advanced gastric cancer and had been diagnosed with pathological stage II or III disease [6]. Based on the ACTS-GC trial, S-1 adjuvant chemotherapy became the standard treatment for patients with stage II and III gastric cancer. This trial suggested that S-1 could improve patient survival by inhibiting peritoneal metastases. Therefore, it seems that prognostic factors might be altered following effective S-1 adjuvant chemotherapy.
In this study, we investigated the prognostic factors for patients with stage II and III gastric cancer who underwent D2 gastrectomy followed by adjuvant chemotherapy with S-1.
Patients and methods
Patients
The patients were selected from the prospective database of the Kanagawa Cancer Center, Department of Gastrointestinal Surgery, Yokohama, Japan, according to the following criteria: (1) histologically proven gastric adenocarcinoma; (2) patients underwent a curative D2 resection for gastric cancer as a primary treatment between June 2002 and March 2010; (3) stage IIA, IIB, IIIA, IIIB, or IIIC disease was diagnosed pathologically according to the Japanese classification of gastric carcinoma 14th edition published by the Japanese Gastric Cancer Association [7]; (4) patients received adjuvant S-1 chemotherapy after surgery at a starting dose of 80 mg/m2/day.
Following the rule defined by the protocol of the ACTS-GC trial, patients received S-1 chemotherapy and were followed at outpatient clinics [6]. Written informed consent was obtained from each patient prior to treatment initiation. Survival data were obtained from hospital records or from the city registry system.
Measurement of tumor diameter
Tumor diameter was measured according to the Japanese classification of gastric carcinoma, 14th edition published by the Japanese Gastric Cancer Association [7]. The resected specimen was opened along the greater curvature to observe the mucosal surface clearly. The opened stomach was placed on a flat board, and the longest tumor diameter was measured and used in the analysis.
Evaluation and statistical analyses
The overall survival (OS) was evaluated by univariate and multivariate analyses. The survival curves were calculated using the Kaplan–Meier method and compared by the log-rank test. Cox’s proportional hazard model was used to perform univariate and multivariate survival analyses. A P value of <0.05 was defined to be statistically significant.
An SPSS software package (v11.0J Win; SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
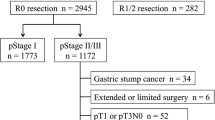
A total of 240 patients underwent surgical resection and were diagnosed with stage IIA, IIB, IIIA, IIIB, or IIIC disease pathologically. Among them, 82 patients were eligible for the present study. All patients had received S-1 as the standard therapy after 2007, when the results of the ACTS-GC trial were presented, or as the test treatment in clinical trials of ACTS-GC or the stomach cancer adjuvant multi-institutional trial group (SAMIT) study. Patients who had received other chemotherapy in other clinical trials and those who did not receive adjuvant chemotherapy were excluded. The patients’ ages ranged between 36 and 80 years (mean 62.0). Fifty-six patients were male, and 26 were female. The pathological stage was IIA in 1 patient, IIB in 23 patients, IIIA in 10 patients, IIIB in 23 patients, and IIIC in 25 patients. The median follow-up period was 24.2 months (range 2.8–76.5 months). The median duration of adjuvant S-1 administration was 7.6 months (range 0.2–34.8 months). The S-1 treatment was continued for 1–3 months in 74 patients, 3–6 months in 61 patients, and 6–12 months in 47 patients. Three patients continued treatment for more than 13 months at the patient’s request. When OS, stratified by clinical factors, was compared by the log-rank test, a significant difference was observed in regard to macroscopic tumor diameter and the depth of tumor invasion (Table 1). Lymph node metastasis was marginally significant. A macroscopic tumor diameter of 70 mm was regarded as the optimal critical point of classification, considering the 3-year survival rate, which was regarded as more reliable than the 5-year survival rate because median follow-up was only 24.2 months. Each clinicopathological factor was categorized, as shown in Table 2, and was analyzed for prognostic significance. Univariate analyses for OS demonstrated that macroscopic tumor diameter was a significant prognostic factor, but that tumor depth and nodal metastasis were only marginally significant (Table 2). Macroscopic tumor diameter was selected for the final model to be analyzed by multivariate analysis (Table 3). The 5-year survival was 64.9% in patients with a macroscopic tumor diameter <70 mm, and it was 33.1% in those with a macroscopic tumor diameter ≥70 mm (Fig. 1).
Discussion
In this report, we first evaluated the potential prognostic factors in stage II/III gastric cancer patients who underwent D2 gastrectomy followed by adjuvant S-1 chemotherapy, and clarified that macroscopic tumor diameter was the most important prognostic factor, based on the hazard ratio and p values.
Some authors have reported the significance of the macroscopic tumor diameter in the prognosis of gastric cancer patients. For example, Kunisaki et al. [8] examined 1215 patients with gastric cancer and classified them into groups with smaller tumors and those with larger tumors, by setting 100 mm as the cutoff value for the maximal tumor diameter. They found that OS was markedly different between stage II/III patients with smaller and larger tumors. Saito et al. [9] evaluated 1473 patients with gastric cancer and divided them into two groups using a cutoff value of 80 mm for the tumor size. They found that the prognosis of patients with the large tumors was significantly worse than the prognosis for those with the small tumors. However, these reports only examined patients who had undergone surgery only, or those who had undergone surgery with adjuvant therapy of unproven efficacy. In the present study, evaluating patients who received S-1 adjuvant chemotherapy, we set the cutoff value for tumor size at 70 mm, considering the 3-year survival rate, and found that tumor size was a strong independent prognostic factor. The optimal cutoff value was different between the previous reports and the present one, which may be explained by the use of S-1 adjuvant chemotherapy in our study; by differences in the durations of the follow-up periods and the numbers of patients; and by inter-institutional variability.
Previously, the depth of tumor invasion had been considered to be the key prognostic factor in gastric cancer patients who underwent curative resection [4, 5]. Several authors indicated that serosal invasion correlated with peritoneal recurrence and a poorer prognosis. In the ACTS-GC trial, the incidence of peritoneal recurrence was 11.2% in the S-1 group and 15.8% in the surgery-only group (P = 0.009) [6]. On the other hand, the incidence of hematogenous recurrence was 10.2% in the S-1 group and 11.3% in the surgery-only group. These results suggest that S-1 was more effective in reducing peritoneal recurrence than in reducing hematogenous recurrence. The depth of tumor invasion might no longer be a useful prognostic factor, because S-1 can reduce the incidence of peritoneal recurrence.
Lymph node metastasis has also been considered as a strong prognostic factor in gastric cancer patients [2, 3]. The ACTS-GC trial demonstrated that hazard ratios for death were better in N0 and N1 than in N2 patients. In the present study, nodal metastasis was found to be a marginally significant factor according to our univariate analysis, and it remained in the final model, but did not reach statistical significance by multivariate analysis. Our results suggest that nodal metastasis may be an inferior prognostic factor compared to the tumor size when the examination is limited to patients who receive S-1 chemotherapy. However, the marginal significance might become more important if the number of patients is increased or if there is longer-term follow-up.
There were many limitations in this study. First, this was a retrospective single-center study with a small sample size. Our findings in this series may have been observed by chance only. Second, the median follow-up period was only 24.2 months, which is not enough to lead to a definite conclusion. Third, the optimal tumor size cutoff value is unclear. In our study, the cutoff value was set at 70 mm by considering the 3-year survival rate. However, regardless of whether the cutoff value was 70, 80, or 90 mm, tumor size remained an independent significant prognosticator (data not shown). Thus, large tumors seemed to have a poor prognosis. An appropriate cutoff value should be determined in other validation studies. Fourth, the depth of tumor invasion and nodal metastasis had prognostic impact in the ACTS-GC study although the tumor size was not examined. When comparing the ACTS-GC trial and our present study, there are some differences in the backgrounds of the patients. The depth of invasion was deeper in the present study (pT4a, pT4b, 61/82; 74.3%) than in the ACTS-GC trial (pT4a, pT4b, 239/529; 45.1%). The incidence of nodal metastases was higher in the ACTS-GC trial (478/529; 90.4%) than in the present study (68/82; 82.9%), while that of TNM-N3 was higher in the present study (29/82; 35.%) than in the ACTS-GC trial (147/529; 27.8%). Because many patients in the present series received S-1 adjuvant chemotherapy as a test arm of the SAMIT trial (a 2 × 2 phase III trial for surgical serosa-positive disease), the incidence of T4a and N3 may be high in this series. Also, differences in background factors could affect prognosticators in stage II/III disease. Considering these limitations, our results should be validated in different series with large sample sizes and sufficient follow-up periods.
In conclusion, the macroscopic tumor diameter was found to be the only significant independent prognostic factor in patients who underwent D2 gastrectomy followed by adjuvant S-1 chemotherapy. Therefore, it appears that the value of prognostic factors can be altered by the use of effective adjuvant chemotherapy.
References
Ohtsu A, Yoshida S, Saijo N. Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol. 2006;24:2188–96.
Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 2000;89:255–61.
Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Prediction of early and late recurrence after curative resection for gastric carcinoma. Cancer. 1996;77:2445–8.
Bozzetti F, Bonfanti G, Morabito A, Bufalino R, Menotti V, Andreola S, et al. A multifactorial approach for the prognosis of patients with carcinoma of the stomach after curative resection. Surg Gynecol Obstet. 1986;162:229–34.
Maruyama K. The most important prognostic factors for gastric cancer patients: a study using univariate and multivariate analyses. Scand J Gastroenterol. 1987;22:63–8.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 14th Japanese ed. Tokyo: Kanehara-shuppan; 2010. p. 5–17.
Kunisaki C, Makino H, Takagawa R, Oshima T, Nagano Y, Kosaka T, et al. Tumor diameter as a prognostic factor in patients with gastric cancer. Ann Surg Oncol. 2008;15:1959–67.
Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Oro S, et al. Macroscopic tumor size as a simple prognostic indicator in patients with gastric cancer. Am J Surg. 2006;192:296–300.
Acknowledgments
This work was supported, in part, by the Kanagawa Health Foundation.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aoyama, T., Yoshikawa, T., Watanabe, T. et al. Macroscopic tumor size as an independent prognostic factor for stage II/III gastric cancer patients who underwent D2 gastrectomy followed by adjuvant chemotherapy with S-1. Gastric Cancer 14, 274–278 (2011). https://doi.org/10.1007/s10120-011-0038-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-011-0038-0