Abstract
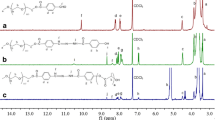
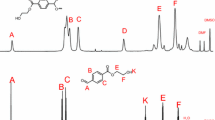
Poly(2-oxazoline) (POx) is a kind of polymeric amides that can be viewed as conformational isomers of polypeptides with excellent cyto- and hemo-compatibility, and is promising to be used as drug carriers. However, the drug loading capacity (DLC) of POx for many drugs is still low except several hydrophobic ones including paclitaxel (PTX). Herein, we prepared a series of amphiphilic POx block copolymers with various functional groups, and investigated the relationship between functional structures and the DLC. Functional POxs with benzyl, carboxyl, and amino groups in the side-chain were synthesized based on a poly(2-methyl-2-oxazoline)-block-poly(2-butyl-2-oxazoline-co-2-butenyl-2-oxazoline) (PMeOx-P(nBuOx-co-ButenOx), PMBEOx) precursor, followed by click reaction between vinyl and the 2-phenylethanethiol, thioglycolic acid and cysteamine. Using thin-film hydration method, eight commonly used drugs with various characteristics were encapsulated within these functional POx polymers. We found that amine-containing drugs were more easily encapsulated by POx with carboxyl groups, while amine functionalities in POx enhanced the loading capacity of drugs with carboxyl groups. In addition, π-π interactions resulted in enhanced DLC of most drugs, except several hydrophobic drugs with aromatic to total carbon ratios less than 0.5. In general, we could successfully encapsulate all the selected drugs with a DLC% over 10% using properly selected functional POxs. The above results confirm that the DLC of polymeric carriers can be adjusted by modifying the functional groups, and the prepared series of functional POxs provide an option for various drug loadings.
Similar content being viewed by others
References
Lasic, D. D. Doxorubicin in sterically stabilized liposomes. Nature 1996, 380, 561–562.
Couvreur, P.; Puisieux, F. Nanopartiles and microparticles for the delivery of polypeptides and proteins. Adv. Drug Deliv. Rev. 1993, 10, 141–162.
Akiyoshi, K.; Kobayashi, S.; Shichibe, S.; Mix, D.; Baudys, M.; Kim, S. W.; Sunamoto, J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J. Control. Rel. 1998, 54, 313–320.
Allemann, E.; Gurny, R.; Doelker, E. Drug-loaded nanoparticles — preparation methods and drug targeting issues. Eur. J. Pharm. Biopharm. 1993, 39, 173–191.
Duncan, R. Drug polymer conjugates—potential for improved chemotherapy. Anti-Cancer Drugs 1992, 3, 175–210.
Jiang, G. B.; Geng, B. W.; Fang, Y. S.; Gao, Y. F. Polymeric micelles as new drug carriers. Modern Chem. Ind. 2007, 27, 69.
Li, Y.; Kwon, G. S. Micelle-like structures of poly(ethylene oxide)-block-poly(2-hydroxyetbyl aspartamide)-methotrexate conjugates. Colloid Surf. B-Biointerfaces 1999, 16, 217–226.
Li, Y.; Kwon, G. S. Methotrexate esters of poly(ethylene oxide)-block-poly(2-hydroxyethyl-L-aspartamide). Part I: effects of the level of methotrexate conjugation on the stability of micelles and on drug release. Pharm. Res. 2000, 17, 607–611.
Gref, R.; Minamitake, Y.; Peracchia, M. T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603.
La, S. B.; Okano, T.; Kataoka, K. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(β-benzyl L-aspartate) block copolymer micelles. J. Pharm. Sci. 1996, 85, 85–90.
Cao, T.; Munk, P.; Ramireddy, C.; Tuzar, Z.; Webber, S. E. Fluorescence studies of amphiphilic poly(methacrylic acid)-block-polystyrene-block-poly(methacrylic acid) micelles. Macromolecules 1991, 24, 6300–6305.
Kwon, G.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Block copolymer micelles for drug delivery: loading and release of doxorubicin. J. Control. Rel. 1997, 48, 195–201.
Kwon, G. S.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Physical entrapment of adriamycin in ab block-copolymer micelles. Pharm. Res. 1995, 12, 192–195.
Piskin, E.; Kaitian, X.; Denkbas, E. B.; Kucukyavuz, Z. Novel PDLLA/PEG copolymer micelles as drug carriers. J. Biomater. Sci.-Polym. Ed. 1995, 7, 359–373.
Ma, S.; Song, W.; Xu, Y.; Si, X.; Zhang, D.; Lv, S.; Yang, C.; Ma, L.; Tang, Z.; Chen, X. Neutralizing tumor-promoting inflammation with polypeptide-dexamethasone conjugate for microenvironment modulation and colorectal cancer therapy. Biomaterials 2020, 232.
Ma, S.; Song, W.; Xu, Y.; Si, X.; Zhang, Y.; Tang, Z.; Chen, X. A ROS-responsive aspirin polymeric prodrug for modulation of tumor microenvironment and cancer immunotherapy. CCS Chem. 2020, 390–400.
Jones, M. C.; Leroux, J. C. Polymeric micelles—a new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111.
Woodle, M. C.; Engbers, C. M.; Zalipsky, S. New amphipatic polymer lipid conjugates forming long-circulating reticuloendothelial system-evading liposomes. Bioconjugate Chem. 1994, 5, 493–496.
Bayley, D.; Sancho, M. R.; Brown, J.; Brookman, L.; Petrak, K.; Goddard, P.; Steward, A. Soluble polymeric carriers for drug delivery. 6. preparation and biodistribution of N5-hydroxyethyl-l-glutamine-co-l-glutamic acid copolymers in rats. J. Bioact. Compat. Polym. 1993, 8, 51–68.
He, Z. J.; Wan, X. M.; Schulz, A.; Bludau, H.; Dobrovolskaia, M. A.; Stern, S. T.; Montgomery, S. A.; Yuan, H.; Li, Z. B.; Alakhova, D.; Sokolsky, M.; Darr, D. B.; Perou, C. M.; Jordan, R.; Luxenhofer, R.; Kabanov, A. V. A high capacity polymeric micelle of paclitaxel: Implication of high dose drug therapy to safety and in vivo anticancer activity. Biomaterials 2016, 101, 296–309.
Lorson, T.; Lubtow, M. M.; Wegener, E.; Haider, M. S.; Borova, S.; Nahm, D.; Jordan, R.; Sokolski-Papkov, M.; Kabanov, A. V.; Luxenhofer, R. Poly(2-oxazoline)s based biomaterials: a comprehensive and critical update. Biomaterials 2018, 178, 204–280.
Luxenhofer, R.; Schulz, A.; Roques, C.; Li, S.; Bronich, T. K.; Batrakova, E. V.; Jordan, R.; Kabanov, A. V. Doubly amphiphilic poly(2-oxazoline)s as high-capacity delivery systems for hydrophobic drugs. Biomaterials 2010, 31, 4972–4979.
Han, Y. C.; He, Z. J.; Schulz, A.; Bronich, T. K.; Jordan, R.; Luxenhofer, R.; Kabanov, A. V. Synergistic combinations of multiple chemotherapeutic agents in high capacity poly(2-oxazoline) micelles. Mol. Pharm. 2012, 9, 2302–2313.
Wan, X. M.; Min, Y. Z.; Bludau, H.; Keith, A.; Sheiko, S. S.; Jordan, R.; Wang, A. Z.; Sokolsky-Papkov, M.; Kabanov, A. V. Drug combination synergy in worm-like polymeric micelles improves treatment outcome for small cell and non-small cell lung cancer. ACS Nano 2018, 12, 2426–2439.
Hwang, D.; Ramsey, J. D.; Makita, N.; Sachse, C.; Jordan, R.; Sokolsky-Papkov, M.; Kabanov, A. V. Novel poly(2-oxazoline) block copolymer with aromatic heterocyclic side chains as a drug delivery platform. J. Control. Rel. 2019, 307, 261–271.
Harada, A.; Kataoka, K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block-copolymers with poly(ethylene glycol) segments. Macromolecules 1995, 28, 5294–5299.
Harada, A.; Kataoka, K. Formation of stable and monodispersive polyion complex micelles in aqueous medium from poly(L-lysine) and poly(ethylene glycol)-poly(aspartic acid) block copolymer. J. Macromol. Sci. Pure Appl. Chem. 1997, A34, 2119–2133.
Kataoka, K.; Ishihara, A.; Harada, A.; Miyazaki, H. Effect of the secondary structure of poly(L-lysine) segments on the micellization in aqueous milieu of poly(ethylene glycol) poly(L-lysine) block copolymer partially substituted with a hydrocinnamoyl group at the N-ε-ootitinn. Macromolecules 1998, 31, 6071–6076.
Yokoyama, M.; Fukushima, S.; Uehara, R.; Okamoto, K.; Kataoka, K.; Sakurai, Y.; Okano, T. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J. Control. Rel. 1998, 50, 79–92.
Li, Q. S.; Yang, Y.; Hong, H.; Chen, X. S. Nanoscaled poly(L-glutamic acid)/doxorubicin-amphiphile complex as pH-responsive drug delivery system for effective treatment of nonsmall cell lung cancer. ACS Appl. Mater. Interfaces 2013, 5, 1781–1792.
Lv, S.; Li, M.; Tang, Z.; Song, W.; Sun, H.; Liu, H.; Chen, X. Doxorubicin-loaded amphiphilic polypeptide-based nanoparticles as an efficient drug delivery system for cancer therapy. Acta Biomater. 2013, 9, 9330–9342.
Shi, Y.; van Steenbergen, M. J.; Teunissen, E. A.; Novo, L.; Gradmann, S.; Baldus, M.; van Nostrum, C. F.; Hennink, W. E. π-π Stacking increases the stability and loading capacity of thermosensitive polymeric micelles for chemotherapeutic drugs. Biomacromolecules 2013, 14, 1826–1837.
Lv, S. X.; Wu, Y. C.; Cai, K. M.; He, H.; Li, Y. J.; Lan, M.; Chen, X. S.; Cheng, J. J.; Yin, L. C. High drug loading and sub-quantitative loading efficiency of polymeric micelles driven by donor-receptor coordination interactions. J. Am. Chem. Soc. 2018, 140, 1235–1238.
Hahn, L.; Luebtow, M. M.; Lorson, T.; Schmitt, F.; Appelt-Menzel, A.; Schobert, R.; Luxenhofer, R. Investigating the influence of aromatic moieties on the formulation of hydrophobic natural products and drugs in poly(2-oxazoline)-based amphiphiles. Biomacromolecules 2018, 19, 3119–3128.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 51673185, 51973215, 51673189, 51829302, 52003268 and 52025035), as well as the support from the Youth Innovation Promotion Association of Chinese Academy of Sciences (No. 2020232).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Information
10118_2021_2547_MOESM1_ESM.pdf
Functional Amphiphilic Poly(2-oxazoline) Block Copolymers as Drug Carriers: the Relationship between Structure and Drug Loading Capacity
Rights and permissions
About this article
Cite this article
Dong, S., Ma, S., Liu, ZL. et al. Functional Amphiphilic Poly(2-oxazoline) Block Copolymers as Drug Carriers: the Relationship between Structure and Drug Loading Capacity. Chin J Polym Sci 39, 865–873 (2021). https://doi.org/10.1007/s10118-021-2547-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-021-2547-6