Abstract
A literature review was conducted to investigate marine global and local extinctions and their drivers; the review followed the PRISMA-EcoEvo guidelines. The data extracted was enhanced with status assessments from the IUCN Red List. We recorded local extinctions for 717 species, of which 18 were global extinctions. Most of these extinctions were recorded on very localized and sub-ecoregion scales. The taxonomic group with the most reported local extinctions was molluscs (31%), followed by cnidarians (22%), fish (17%) and macroalgae (15%). The dominant drivers of extinction differed by taxonomic group. High mobility taxa were driven extinct mainly by overexploitation, whereas low mobility taxa from pollution, climate change and habitat destruction. Most of these extinctions were recorded in the Temperate Northern Atlantic (41%) and the Central Indo-Pacific (30%). Overexploitation was historically the primary driver of marine local extinctions. However, in the last three decades, other drivers, such as climate change, climate variability, and pollution, have prevailed in the published literature. Half of the reported extinctions were of species not assessed by the IUCN Red List, and 16% were species in threatened categories. Global extinctions in the marine environment were mainly attributed to overexploitation, followed by invasive species, habitat destruction, trophic cascades, and pollution. Most extinctions reported in the literature were derived from low-confidence data. Inadequate monitoring may lead to false reports of extinctions or silent extinctions that are never reported. Improved conservation and restoration actions are urgently needed to halt biodiversity loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As humanity traverses into the Anthropocene, the biodiversity crisis exacerbates (O’Hara et al. 2021; Cowie et al. 2022). Modern-day species extinction rates are increasing and are about a hundred to a thousand times higher than natural background extinction rates (Ceballos et al. 2015; Rounsevell et al. 2020). These rates are driven mainly by terrestrial extinctions, as marine extinctions are substantially lower (McCauley et al. 2015). Human-caused global extinctions may be few in the oceans (IUCN 2022), but there is a significant record of local, ecological, and commercial extinctions in marine realms (Dulvy et al. 2003; McCauley et al. 2015). These extinctions have detrimental impacts on ecosystem functioning and services (Worm et al. 2006; Cardinale et al. 2012).
Historically marine biodiversity was in peril mainly by overexploitation and habitat destruction (Dulvy et al. 2009). Albeit we are still observing overexploitation and habitat loss threatening marine biodiversity, improved management has, to some extent, mitigated the effect of these two drivers. At the same time, the impacts of climate change and pollution are at their peak (Duarte et al. 2020). Fishing evolved to be an activity that in a year can consume 19 billion kWh of energy, corresponding to covering the distance to the Moon and back 600 times (Kroodsma et al. 2018). Exploitation has pushed marine mammals, sharks, and bony fishes to the verge of extinction and depleted many populations (Springer et al. 2003; Worm and Tittensor 2011; Yan et al. 2021). Coastal development, trawling, and dredging can result in habitat loss and destruction of spawning and feeding grounds resulting in extinctions of anadromous fishes (Gustafson et al. 2007), seagrass and macroalgae in shorelines of coastal cities (Waycott et al. 2009), and many other taxa (Dulvy et al. 2003). In the last decades, we have been witnessing climate change triggering collapses and range contractions of populations, especially at the trailing-warmer edge of species distribution (Rilov 2016; Wernberg et al. 2016), and in some cases, the poleward expansion of populations with negative impacts on the native ecosystems and human well-being (Pecl et al. 2017). Pollution in the marine environment imperils biodiversity through marine litter, hazardous pollutants, oil spills, and urban waste, driving many populations locally extinct (Cadée et al. 1995; Phillips and Blackshaw 2011; Poquita-Du et al. 2019). Other stressors such as invasive species, trophic cascades or even natural causes also threaten marine biodiversity and are blamed for marine population collapses (Myers et al. 2007; Nehru and Balasubramanian 2018; Tsirintanis et al. 2022). These stressors can interact, and the cumulative impact on biodiversity may be amplified (Rasher et al. 2020; Gissi et al. 2021).
Identifying extinctions of populations is important for conservation management for multiple reasons. We can understand which species and populations must be prioritized for protection, which traits make species vulnerable to extinction, and what went wrong in conservation management (Dulvy et al. 2009). However, not all extinctions are the same; the spatial extent, the driver of extinction, or the recolonization ability of the species can differ for each extinction case. Also, not all reports of extinctions are reliable, as the methods used or the detectability of the species can vary; thus, some populations may be wrongfully stated as extinct. Fallacious declarations of species or population extinctions can damage the credibility of conservation scientists, causing policy-makers to mistrust conservation experts. Additionally, false extinction reports can expose a species or a population in need of protection to non-protected status after the species or population is considered extinct (Monte-Luna et al. 2007; Cowie et al. 2022). Understanding the differences among extinction reports and mapping them is crucial in conservation biology.
This literature review aimed to identify marine global and local extinctions and their possible drivers. Conscious that some of these reports may be wrong due to insufficient evidence, we followed a more indulgent approach according to the precautionary principle (Kriebel et al. 2001). In this framework, this literature review provides essential spatiotemporal information about marine extinctions and their drivers. The validity of the studies reporting extinctions and their drivers was also critically appraised. This information is crucial, as it can help identify inadequacies in conservation management and help policymakers and marine managers set priorities. However, reported published data was insufficient to conduct a thorough assessment of each extinction case to confirm its veracity.
Methodology
A global literature review was conducted on local and global extinctions and their drivers in the marine environment, applying the PRISMA-EcoEvo approach (Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Ecology and Evolutionary Biology; Moher et al. 2009; O’Dea et al. 2021; see Supplementary material 1 for PRISMA Eco-Evo checklist). We searched the scientific literature through the Scopus search engine in June 2022 and again in February 2023. Search criteria included at least one term among ‘marine’, ‘ocean’, ‘sea’, ‘coastal’, ‘pelagic’, ‘*tidal’, ‘bay’, and ‘gulf’ and at least one term among ‘local extinction’, ‘population extinction’, ‘species extinction’, ‘regional extinction’, ‘local collapse’, ‘population collapse’, ‘species collapse’, ‘regional collapse’, ‘local contraction’ and ‘extirpation’. We did not put any restrictions on the year of publication. To narrow down the initial search results, we filtered the given results only to relevant subject areas and journals. We also considered only English language studies. The literature search resulted in 2,297 articles (Fig. 1). Additionally, we identified 112 relevant articles not found in the initial search through the reference lists of selected articles and added them for screening.
To be included in our review, studies needed to meet the following inclusion criteria: only studies reporting local or global extinctions in the marine environment were included; studies concerning extinctions before the Holocene were excluded; studies addressing local disappearance of dynamic populations which undergo periodic local extinctions (e.g., extinctions and recolonisations in the intertidal zone) were excluded; local extinctions of alien species (sensu Essl et al. 2018) in their invasive range were not included. According to our inclusion and exclusion criteria, the collected papers were screened by the first author (AN) based on title and abstract (n=2,390). For articles that passed the first-stage screening, the full text was retrieved and assessed for inclusion, based on the same inclusion and exclusion criteria (n=638). Overall, 194 articles passed full-text screening and met the eligibility criteria (Fig. 1). For included articles, the data extracted from the full text (by AN) were: authors, year of publication, country, marine realm (based on Spalding et al. 2007), species, last sighting of extinct species, and the driver of extinction. Also, information on the spatial extent of the extinction was extracted, defined as very localized (e.g., bays, coastal cities shorelines, small oceanic islands), sub-ecoregion scale (similar order of magnitude to ecoregion scale but still smaller), ecoregion scale, extensive (spanning more than one ecoregion), and global. Drivers of extinctions were classified into seven predefined categories, i.e. habitat destruction, overexploitation (including bycatch), climate change, invasive species, trophic interactions and cascades, pollution, climate variability, diseases and parasites; other drivers identified were grouped. The detection method of the extinction, direct or indirect, was recorded. We considered direct detection methods ecological monitoring programs which are species or taxa-specific, whereas indirect methods are based on comparisons with historical biodiversity lists, other studies, or sub-fossils and archaeological findings (Dulvy et al. 2003). For each local extinction, we extracted the temporal coverage of the monitoring program or the species’ occurrence observations. If the extinction was detected with a direct method, we defined low, medium, or high temporal coverage based on the number of surveys conducted (2 for low, 3-4 for medium, >5 for high). When the method was indirect, if data were available, we considered for temporal coverage the number of species records from different surveys-sources or the number of fossil findings (1-2 records are considered low coverage, 3-4 medium, >5 high). For each extinction case, the time frame of the study was extracted and categorized either as <10 years or ≥10 years. Lastly, the type of evidence for the driver of the extinction was extracted. Six categories were used based on Katsanevakis et al. (2014): manipulative experiments, natural experiments, direct observations of impact, modelling, non-experimental-based correlations, and expert judgment.
We critically appraised the recorded extinctions based on five retrieved variables, including the detection method, the temporal coverage, the time frame of the study, the type of evidence for the identified driver, and the size of the extinction area. Reported extinctions of high confidence are characterised by long-term ecological monitoring and monitoring methods that secure high detectability. Direct detection through ecological monitoring is typically conducted by expert personnel and with scientific methodologies, which often try to handle imperfect detectability (Katsanevakis et al. 2012). On the other hand, indirect detection methods with low temporal coverage may provide less reliable indications of true absence (Hortal et al. 2008). Possible inaccuracies in species identification of the species and low detection power of the different methods-sources used, which may occur with indirect detection methods, can lead to false assessments. Additionally, an important aspect of this review and extinction reports, in general, is to investigate the drivers of extinction. Attributing an extinction specifically to one or more drivers often needs rigorous assessments over a large time frame and spatial extent combined with strong inference evidence (Cooley et al. 2022). To synthesize this information into an overall validity index, we created a scoring system (Table 1). Extinction cases were assigned a score between zero and two for all five appraisal variables. If data for one of the appraisal variables were unavailable, its score was set to zero. This decision was made because missing important aspects of an extinction report reduce the overall validity of the report. The summed score of all five variables for each extinction case was then transformed into a three-level categorical variable indicating an overall validity index. The summed scores may range from zero to ten; scores from zero to three were categorized as low validity extinction cases, scores from four to six as medium, and scores from seven to ten as high.
We collected data on the status and date of assessment from the IUCN Red List for species that were recorded suffering local or global extinction. For globally extinct species, we searched the latest scientific literature to identify concordance or discordance between IUCN Red List assessments and scientific articles. Augmenting the results with data from the IUCN Red List can inform conservation biologists and policymakers while also highlighting potential flaws in species status assessment.
Descriptive information is presented for the data collected. In cases of missing data, such as the date of the last sighting or unreported driver of extinction, we excluded the missing values when calculating descriptive statistics of the specific variables. Using chi-square tests, we conducted cross-tabulation analyses to determine whether there were significant associations between selected pairs of variables. Following the empirical rule of >5 for the expected frequencies, we grouped specific categories for each variable when needed. We conducted this analysis in R Programming Software (RStudio Team, Version 4.1.0, 2022).
Results
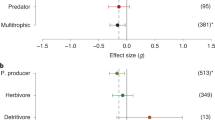
We recorded local extinctions for 717 marine species. Some of these species had multiple local extinctions in different geographical areas, such as the giant clam (Tridacna gigas) with nine local extinctions, the largetooth sawfish (Pristis pristis) with five local extinctions, the coral Seriatopora hystrix with five local extinctions, and the macroalga Sargassum hornschuchii with four local extinctions (the complete list of local extinctions recorded is given in Supplementary material 2). In 56% of the cases, the recorded extinctions were very localized, followed by sub-ecoregional (35%), ecoregional (4%), extensive (2%), and global extinctions (2%). The taxonomic group with the highest number of extinctions was Mollusca, with 31% of all recorded extinctions, followed by Cnidaria (corals; 22%), Macroalgae (15%), Osteichthyes (12%) and Chondrichthyes (5%); other taxonomic groups collectively covered 15% of the recorded extinctions (Fig. 2). Chondrichthyes was the taxonomic group with the most ecoregional and extensive extinctions (43% of all cases), molluscs in sub-ecoregional cases (47%), and cnidarians in localized extinctions (33%) (See Figure S1 in Supplementary material 3).
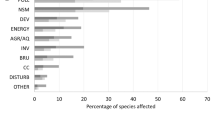
Pollution was the most frequent driver of marine extinctions, reported 302 times, followed by climate change (273), habitat destruction (226), overexploitation (185), climate variability (144), trophic cascades (91), diseases and parasites (32), invasive species (27), and 34 other cases (natural processes, natural disasters, and HAB’s) (Fig. 3). Among taxonomic groups, extinction drivers differed significantly (chi-square test; keeping the four most frequent taxonomic groups and grouping all others; keeping the four most frequent reported drivers and grouping all others; p<0.001) (See Figure S2a in Supplementary material 3). Climate change, pollution, and habitat destruction were the main drivers of extinction for Mollusca (39%, 25% and 24%, respectively), climate variability and climate change for Cnidaria (32% and 31%), overexploitation for Osteichthyes (46%), Chondrichthyes (87%), Mammals (74%), and Aves (62%), and pollution (53%) for Macroalgae (Fig. 2). The study results reveal a significant increase in local extinctions in recent decades (Fig. 4). Temporal evolution of the reported drivers reveals that until the mid-1990s, overexploitation, pollution, and habitat destruction were the primary drivers of extinctions. From the late 1990s and onwards, reports that attribute marine extinctions to climate change and climate variability have substantially increased.
The majority of reported extinctions were concentrated in the Temperate Northern Atlantic (41% of the total extinctions) and Central Indo-Pacific (30%), followed by Tropical Atlantic (9%), Western Indo-Pacific (7%), Temperate Northern Pacific (4%), Eastern Indo-Pacific and Temperate Australasia (3%). Extinctions were less common in other realms, and no local extinctions were reported in the Southern Ocean (Fig. 5). The reported possible drivers of extinctions differed significantly by realm (chi-square test; keeping the five most frequent realms and grouping all others; keeping the four most frequent drivers and grouping all others; p < 0.001) (See Figure S2b in Supplementary material 3). Among the five realms with the most reported extinction cases, the relative importance of overexploitation was the highest in the Temperate Northern Pacific and Tropical Atlantic, climate change in the Temperate Northern Atlantic, pollution in the Central Indo-Pacific, and climate variability in the Western Indo-Pacific (Fig. 6). The frequency of reported extinctions between temperate and tropical realms differed significantly by taxonomic group (chi-square test; grouping realms into tropical and temperate areas; keeping the ten most frequent taxonomic groups and grouping all others; p < 0.001) (See Figure S3a in Supplementary material 3). Extinctions of cnidarians, mangroves and echinoderms were primarily reported in tropical realms, while macroalgae, mammals, and fishes were mainly reported in temperate realms.
Number of extinctions recorded throughout the different marine Realms (sensu Spalding et al. 2007)
We have identified 18 global marine extinctions, which were highly conclusive based on the literature reviewed (Table 2). Exclusions of some global extinction cases were based on the controversial status of the species. While the IUCN Red List reports 21 marine species as globally extinct, we found evidence against four of these extinctions and evidence for one global extinction that was not included in the IUCN Red List. Littoraria flammea is listed as globally extinct on the Red List based on a 1996 assessment (Bouchet 1996a) but was later found alive in its native habitat by Dong et al. (2015). The extinction of another mollusc species, Lottia edmitchelli, is being questioned as to whether it was a human-caused Holocene extinction (Cowie et al. 2022; Powell 2022). Omphalotropis plicosa was assessed as extinct in 1996 (Griffiths 1996), but it has been recorded several times recently (Florens and Baider 2007). Lastly, the North Sea Houting (Coregonus oxyrinchus), which is listed as extinct in the Red List (Freyhof and Kottelat 2008), retains a controversial taxonomic status (Jacobsen et al. 2012; Mehner et al. 2018). We also included one global extinction not listed on the IUCN Red List: the endemic New Zealand bird, Leucocarbo septentrionalis, which went extinct around 1450 (Rawlence et al. 2017). The Galapagos Damsel fish (Azurina eupalama) has not been recorded since 1982 (Dulvy et al. 2009), but according to the IUCN Red List assessment, it is Critically Endangered and Possibly Extinct, as more extensive surveys are needed to determine its status (Allen et al. 2010); thus this species was not included.
Of the 18 global extinctions, ten were Aves, four Mammalia, two Osteichthyes, one Macroalga, and one Mollusc (Table 2). Five global extinctions occurred in Temperate Northern Atlantic, four in Temperate Northern Pacific, four in Temperate Australasia, three in Tropical Atlantic, and two in Central Indo-Pacific. The main driver of global marine extinctions was overexploitation (in 13 cases, it was reported as a possible driver), followed by invasive species (seven times reported), habitat destruction (five times reported), trophic cascades (three times reported), and pollution (one time reported). No global marine extinction was attributed to climate change, diseases or other drivers. The Chinese Paddlefish was the latest marine global extinction (Psephurus gladius), last sighted in 2003 (Qiwei 2022).
Most extinction cases were categorized as having low and medium validity, and thus the accuracy of some of these reports is in question (Fig. 3; Figures S4-S8Supplementary material 3). These results indicate that many of these reports are possibly inaccurate either about the true absence of the species in the area or the driver identified. Most Mollusca reported extinctions have low (44%) and medium (51%) validity. Macroalgae extinctions follow a similar pattern, as most cases have low (37%) and medium (61%) validity. Most Cnidaria reported extinctions were mainly of low validity (59%), but a substantial percentage (19%) were classified as high validity. In contrast, most Osteichthyes reported extinctions were high-validity cases (48%). Most Temperate Northern Atlantic extinction cases are of medium validity (63%), with almost equal low and high validity extinction cases. The Central Indo-Pacific, on the other hand, was dominated by low validity cases (65%), with medium (20%) and high (15%) validity cases being less common. The Tropical Atlantic, Western Indo-Pacific and Temperate Northern Pacific had a majority of medium validity cases (51%, 45% and 64%, respectively); however, the Tropical Atlantic and Western Indo-Pacific have substantial low validity cases (both 42%), while the Temperate Northern Pacific has a considerable number of high validity cases (24%).
Most of the extinctions recorded were detected by indirect methods (69%), of which 40% were studies of low temporal coverage (Fig. 7). On the other hand, direct detection methods (31%) were primarily studies of high temporal coverage. The type of evidence for the possible drivers of extinctions identified was mostly expert judgment (62%) and non-experimental correlations (25%), i.e., of low inferential strength (Fig. 7). A fair number of extinction cases did not identify any drivers (9%), while drivers identified by direct observation of impact (3%), manipulative experiments (1%), and modelling (less than 1%) were scarce. We did not find any driver being identified based on natural experiments. Manipulative experiments usually tested the lethality of various drivers and were implemented as in vitro and in vivo experiments (Bryan et al. 1986; Chevaldonné and Lejeusne 2003; Dooley et al. 2013; Smale and Wernberg 2013), sometimes reinforcing non-experimental correlation studies (Rilov 2016; Yeruham et al. 2020), or as in situ transplantation experiments (van Katwijk et al. 2010; Dumbauld et al. 2011). Modelling studies usually explored the drivers of extinction for well-recorded extinctions, where sufficient data were available for modelling approaches, such as population viability analysis (Turvey and Risley 2006; Kayanne et al. 2022).
Sankey diagram representing the frequency of temporal coverage, detection methods and type of evidence (ME stands for manipulative experiment). The Sankey diagram was created with flourish.studio (https://flourish.studio)
About half (53%) of the recorded extinctions were of species that had not been assessed in the IUCN Red List, and 1% were assessed as data deficient. Of the remaining species, 16% were in threatened categories, and 28% were in the near-threatened, conservation-dependent, and least-concern categories. Species considered extinct according to the IUCN Red List accounted for 2% of the total recorded extinctions. It is important to note that we could not draw IUCN Red List assessments for 33 extinction cases because these taxa were not identified at the species level. Species in threatened categories were usually species reported as extinct by studies with high temporal coverage contrary to not evaluated species, mostly reported as extinct by studies with low temporal coverage (Fig. 8).
The mobility of the taxonomic group significantly diversified the drivers of extinctions (chi-square test; high mobile taxonomic groups: Osteichthyes, Chondrichthyes, Mammals, Aves, and Reptiles and all others were grouped into lower mobility taxonomic groups; invasive species and climate variability are grouped with other drivers p < 0.001) (See Figure S3b in Supplementary material 3). High-mobility taxonomic groups were reported to get locally extinct predominantly from overexploitation, whereas low-mobility taxonomic groups from pollution, climate change, climate variability, and habitat destruction. Also, most global, extensive, and ecoregion scale extinctions were of species from taxonomic groups with high mobility (89% for global and extensive, and 66% for ecoregion scale; concerning Chondrichthyes, Osteichthyes, Mammals and Aves), whereas sub-ecoregion and very localized extinctions mainly concerned low mobility taxonomic groups (75% and 88%, respectively).
Discussion
Key findings
The previous effort to assess Holocene extinctions in the sea was conducted two decades ago by Dulvy et al. (2003). The results of this review indicate a sixfold increase in marine local extinctions since Dulvy et al. (2003). Even after 20 years, low-inference data constitutes the majority of available ecological knowledge. Since the last list of marine local extinctions, there has been a notable increase in mollusc and coral extinctions and reports that attribute pollution and climate change as possible extinction drivers.
Extinction characteristics by taxonomic group
The high number of reported molluscs extinctions can be explained mainly by two factors: (1) Mollusca is the taxonomic group with the highest number of described marine species, particularly gastropods and bivalves (Appeltans et al. 2012), and (2) these two classes are easily sampled and stored, and can even be studied from death assemblages (Albano et al. 2021) allowing for easy comparisons between historical and present-day molluscan fauna (van der Meij et al. 2009; Crocetta et al. 2013; Rilov 2016). Although molluscan loss occurs at a very localized and sub-ecoregional scale, it is crucial to identify these losses to understand their causes and mechanisms and to inform marine managers and conservation policymakers. In the eastern Mediterranean, where many molluscan extinctions were recorded, climate change and invasive species give rise to “novel ecosystems”, which are predicted to further expand geographically. It is proposed that conservation management should focus on conserving ecosystem functioning that could be secured by non-native species, as native biodiversity is doomed to largely go extinct (Rilov et al. 2020; Albano et al. 2021). The less in frequency, ecoregional or extensive molluscan extinctions concerned large-sized and emblematic molluscs, such as Pinna nobilis, which went locally extinct in many Mediterranean areas because of an invasive pathogen (Katsanevakis et al. 2022), and giant clams of the family Tridacnidae due to overexploitation (Bin Othman et al. 2010; Mei Lin Neo et al. 2017).
Many cnidarian extinctions of the classes Hexacorallia, Octocorallia and Hydrozoa were recorded. Increasing temperatures, especially observed during marine heatwaves, can dislodge algal symbionts from corals. Zooxanthellae loss leads to coral bleaching, which leaves corals vulnerable and can lead to die-offs if the phenomenon is prolonged (Glynn 1984; Brown 1997). Seemingly, heatwaves impact severely fast-growing, branching, and tabular species triggering shifts to assemblages with less complex three-dimensional structures (Hughes et al. 2018); our results revealed that these types of corals were the most common to have multiple local extinctions (Seriatopora hystrix, Stylophora pistillata, Acropora valida). Climate change impacts are expected to have broad geographical impacts on corals (Freeman et al. 2013), but most recorded coral extinctions were recorded on localized or sub-ecoregional scales. It is possible that the effects of climate change have yet to cause more widespread coral extinctions, given that global warming and marine heatwaves are not geographically homogenous (Lough 2012; Hughes et al. 2018; Garrabou et al. 2022). It is important to note that most large-scale studies have focused on coral cover rather than on species-level contractions due to the lack of comprehensive species-specific datasets on a regional level (Dietzel et al. 2021). This knowledge gap is concerning, as population shifts and regional extinctions can remain undetected.
Except for one case of global extinction, only very localized extinctions have been reported for macroalgae and angiosperms. Like corals, there is a lack of comprehensive, long-term time series for macroalgae and angiosperms at regional scales (Waycott et al. 2009; Krumhansl et al. 2016; Unsworth et al. 2019). Both macroalgae and angiosperms exhibit high natural variability, making it difficult to identify long-term changes. This data gap is perilous as it can lead to unrecorded extinctions and flaws in conservation status assessments and protection prioritization (Richards and Day 2018).
Usually, fish that have gone locally extinct due to overexploitation are high-trophic-level species (Worm and Tittensor 2011; Yan et al. 2021). These extinctions can often occur at large scales, unlike molluscs or corals. The fact that higher trophic level species are going extinct in vast areas must be of deep concern, as cascading effects can have detrimental impacts on whole ecosystems (Estes et al. 2011). For example, local and ecological extinctions of great sharks in the US Atlantic coasts resulted in the collapse of scallop fisheries in North Carolina Bay (Myers et al. 2007).
Marine mammals have an elevated risk of extinction because of their life cycles (Davidson et al. 2012), hence the four global extinctions of marine mammals. Hunting has been regulated or banned, and most depleted populations of marine mammals are recovering (Duarte et al. 2020). However, considering their depleted status and vulnerability, concerns are raised about plastic pollution, ghost fishing, and by-catch caused mortality, which increases through time and remains largely unregulated (Stelfox et al. 2016; Avila et al. 2018; Roman et al. 2021).
Although local extinctions have been recorded for mangroves, the situation is rather optimistic, as conservation measures have succeeded in reducing the loss rate by one order of magnitude (Friess et al. 2020). Maintaining the positive trend for mangroves must be ensured by assessing the threat of sea-level rise and innovative conservation actions in line with adaptive management principles (Katsanevakis et al. 2011).
Drivers of extinction, spatial patterns, and areas of concern
A significant share of marine extinctions has been attributed to climate change in most marine realms. Attributing extinctions to climate change must be accompanied by multiple lines of robust evidence (Cooley et al. 2022). For example, contemporary observations over-decadal time scales on a regional level, combined with experimental studies investigating physiological thresholds of multiple identified stressors in the investigated area, provide a robust understanding of the causes of declines. A good example is the case of the Mediterranean sea urchin Paracentrotus lividus local extinction in Israel. There are contemporary observations of the decline of the species in the area (Rilov 2016) combined with experimental evidence which reveals that ocean warming and competition for resources with an alien herbivore are the leading causes of the decline (Yeruham et al. 2015; Yeruham et al. 2020). However, for most extinction cases, there is a lack of experimental evidence, which hinders a clear interpretation of the results and elaboration of the role of climate change as a driver of extinction in the present era. In tropical realms, extinctions of corals were particularly recorded in the Central-Indo Pacific, considered one of the most important biodiversity hotspots globally (Hughes et al. 2002). This realm is expected to be highly impacted by climate change, causing unsuitable thermal conditions for corals (Descombes et al. 2015). High-risk areas for corals are localities where climate change threatens corals in synergy with other threats such as pollution and trophic cascades, usually urbanized and exploited areas (Hongo and Yamano 2013; Poquita-Du et al. 2019; Richards et al. 2021). Only a few local extinctions have been recorded for the Red Sea and the Persian Gulf corals, and none for the corals in Brazil. However, there is a significant knowledge gap in these areas (Morais et al. 2018), and concurrently they face multiple intense human threats (Amaral and Jablonski 2005; Halpern et al. 2008). Under these conditions, the coral reefs of the Red Sea, the Persian Gulf, and Brazil are also of concern, and more research and ecological monitoring are needed to avoid silent extinctions. Climate change triggers distribution shifts in temperate areas through poleward migration and local extinctions of invertebrates, fishes and macroalgae. Most of the extinctions attributed to climate change in temperate realms have been recorded in the Mediterranean Sea. Recent studies have identified the Mediterranean as a climate change hotspot, as global warming and marine heatwaves are more intense and frequent in this land-locked basin (Pisano et al. 2020; Garrabou et al. 2022). The Mediterranean Sea is also one of the most impacted areas due to multiple anthropogenic pressures such as overexploitation, pollution, and invasive species, especially in the easternmost part (Micheli et al. 2013). The Mediterranean Sea exhibits high endemism (Coll et al. 2010). However, Mediterranean endemic species are very vulnerable as poleward migration outside the Mediterranean can be difficult for many species due to physical and oceanographic barriers, leading to local and global extinctions (Ben Rais Lasram et al. 2010).
Overexploitation has led to the extinction of mainly fishes, particularly along the Atlantic coast of the US and in Europe, specifically in areas such as the North Sea, the Mediterranean and the Black Sea, where fishing effort is high (Kroodsma et al. 2018). However, fishing regulations have been successful in these areas, and fish stocks are expected to improve further, except for the Mediterranean and Black Sea stocks (Hilborn et al. 2020). Overexploitation poses a significant threat, particularly in areas where ineffective fisheries management is accompanied by illegal, unreported, and unregulated fisheries and significant knowledge gaps. This leads to high uncertainties and raises serious concerns for the status of both targeted and non-targeted species affected by such practices (Everett et al. 2015; Bănăduc et al. 2016; Hilborn et al. 2020). Protecting and monitoring nearshore habitat specialists, such as sawfishes and diadromous fish, is crucial. Their life history characteristics and the fact that they are threatened by both overexploitation and habitat destruction make them highly susceptible to extinction. A recent example is the global extinction of the Chinese paddlefish (Qiwei 2022). Sawfishes are highly endangered and have become locally extinct in many tropical and subtropical areas, such as the Central Indo-Pacific, Western Indo-Pacific, Tropical Atlantic, and Southern Africa. Sawfishes are highly vulnerable to human presence as they live near the coast where human pressure is high (Halpern et al. 2008), are overexploited in these areas for their highly prized fins and rostra, are easily caught as by-catch as they get entangled in fishing gear, and vital habitats for their life cycle such as mangroves and estuaries are being degraded (Yan et al. 2021). Mortality on all five species of sawfish can only be reduced through strict protection and mitigation of by-catch and the replenishment of their habitats through protection and restoration actions (Dulvy et al. 2016). Many local extinctions of diadromous fishes were recorded in the US and Europe due to extensive exploitation and river engineering. More effective fisheries management, restoration actions and creation of passages for migration routes have been suggested (Pikitch et al. 2005; Verhelst et al. 2021). Despite significant conservation action to protect and help recover diadromous fish populations, the desired results are usually not achieved (Verhelst et al. 2021), while climate change poses a new additional threat (Lassalle and Rochard 2009). Hence, considering the already conservation-dependent status of these species, research and conservation should evaluate the impacts of climate change and promote adaptive management (Katsanevakis et al. 2011; Rilov et al. 2020).
Critical appraisal of the evidence base
For most marine organisms, ecological monitoring data are generally poor, while the status quo is adverse for biodiversity, so it seems preferable to follow the precautionary principle (Myers 1993) and reveal possible local extinctions, encouraging scientific scrutiny and helping to ensure accurate assessments. Some reported cases may be just functional extinctions that can severely affect the ecosystem (Valiente-Banuet et al. 2015). These cases must also be addressed so conservation policies can be implemented.
The problem of limited knowledge of historical species distribution and population status is crucial and must be dealt with. There are new technologies and innovative and often low-cost approaches that can be implemented to achieve better ecological monitoring and large-scale datasets, such as remote sensing utilizing satellites and aerial or surface drones with high-end cameras and sensors, affordable underwater robotic vehicles and gliders (Peukert et al. 2018; Constantinou et al. 2021; Gazis and Greinert 2021; Maslin et al. 2021), upgraded artificial intelligence algorithms for species recognition (Brook et al. 2020), environmental DNA (Valentini et al. 2016; Marques et al. 2021; Stauffer et al. 2021; Stefanni et al. 2022), and empowering biodiversity observations through citizen science initiatives (Stuart-Smith et al. 2017; Giovos et al. 2019). Also, it is essential that unpublished historical and recent data about species occurrence, especially the ones with a high risk of extinction, are made available to the scientific community.
How to halt biodiversity loss in the marine environment
There are only 18 well-grounded global marine extinctions, but many marine species retain uncertain status. The low number of marine extinctions does not reflect the deteriorated status of the ocean ecosystems. There are marine species today that are probably doomed to global extinction if we do not act swiftly (Albano et al. 2021; Hamilton et al. 2021; Katsanevakis et al. 2022). Many more species have become locally extinct, with the consequences of these losses largely understudied. Assessing the effects of local extinctions across all taxa is a knowledge gap that should be filled by future research. Furthermore, these local biodiversity losses should be recovered to the extent possible. The marine environment is in a critical state, and aiming to sustain the present status is inadequate to achieve the vision of the working group on the post-2020 Global Biodiversity Framework under the Convention on Biological Biodiversity (CBD): “by 2050, biodiversity is valued, conserved, restored and wisely used, maintaining ecosystem services, sustaining a healthy planet and delivering benefits essential for all people”. Rebuilding marine ecosystems ought to be a top priority in the targets set post-2020 (Duarte et al. 2020).
Many extinctions in this review occurred across multiple nations’ borders, and spatial heterogeneity in the research effort was evident. Conservation goals must be accompanied by improved systematic conservation planning within a transboundary framework encouraging international collaboration and the development of coherent networks of marine protected areas (Katsanevakis et al. 2020). The results of this review also highlight an increase in climate change-attributed extinctions, which underlines the need for adaptive management to ensure the success of conservation efforts, as marine conservation is becoming a fast-moving target (Rilov et al. 2019, 2020) due to the temporal and spatial variation of the cumulative human pressures which are intensified by climate change (Gissi et al. 2021; O’Hara et al. 2021). Adaptive management should be informed by evaluating the success of conservation actions, and with continuous status and cumulative effect assessments, following a risk-based approach (Katsanevakis et al. 2011; Stelzenmüller et al. 2018, 2020). Flawless and agile coordination is also needed to monitor the progress globally, resolve inadequacies or adapt new scientific and technological advancements while encouraging the participation of the public and stakeholders to ensure the viability of conservation actions at local and regional scales. Conservation optimism with an evidence-based realism and communicating science positively, focusing on the connection between ocean health and human wellbeing, should be implemented in every type of conservation communication and our narrative about the oceans, as it can unite and encourage collaborations and public support in the roadmap to successful conservation (McAfee et al. 2019; Borja et al. 2022).
Conclusion
This review revealed a growing number of reported marine local extinctions. Extinctions were evident in various taxonomic groups, caused by different drivers and at different spatial scales. The output of this work unravels patterns of extinctions for many taxonomic groups and realms, allowing policymakers to gain insights into the available evidence. Furthermore, policymakers can be informed about where our knowledge is lacking, based on the validity assessments and the highlighted areas of concern, helping to direct science and conservation funds where needed. Assessing the validity of extinctions can also trigger scientific interest in specific extinction cases and encourage more scrutinized scientific investigations. The revealed increasing role of specific extinction drivers, such as climate change, can direct future mitigation efforts.
Evidence of marine extinctions is commonly of low inferential strength. The lack of long-term ecological data and robust evidence about the drivers of extinctions can be highly perilous. Extinctions may go unnoticed, and conservation management efforts may be misdirected. Continuous ecological monitoring, upscaled by innovative and cost-effective tools, is essential for successful biodiversity conservation. At the same time, the complex interactions of multiple stressors on populations need to be disentangled through manipulative experiments and modelling to better understand the drivers of decline. This improved knowledge should respond to the pressures of each region and be evaluated continuously under an adaptive management framework.
References
Albano PG, Steger J, Bošnjak M, Dunne B, Guifarro Z et al (2021) Native biodiversity collapse in the eastern Mediterranean. Proc R Soc B 288:20202469. https://doi.org/10.1098/rspb.2020.2469
Allen G, Robertson R, Rivera R, Edgar G, Merlen G et al (2010) Azurina eupalama. The IUCN Red list of threatened species 2010:e.T184017A8219600. https://dx.doi.org/10.2305/IUCN.UK.2010-3.RLTS.T184017A8219600.en. Accessed 20 Oct 2022
Amaral ACZ, Jablonski S (2005) Conservation of Marine and Coastal Biodiversity in Brazil. Conserv Biol 19:625–631. https://doi.org/10.1111/j.1523-1739.2005.00692.x
Appeltans W, Ahyong ST, Anderson G, Angel MV, Artois T et al (2012) The Magnitude of Global Marine Species Diversity. Curr Biol 22:2189–2202. https://doi.org/10.1016/j.cub.2012.09.036
Avila IC, Kaschner K, Dormann CF (2018) Current global risks to marine mammals: Taking stock of the threats. Biol Conserv 221:44–58. https://doi.org/10.1016/j.biocon.2018.02.021
Baisre JA (2013) Shifting Baselines and The Extinction of The Caribbean Monk Seal. Conserv Biol 27:927–935. https://doi.org/10.1111/cobi.12107
Bănăduc D, Rey S, Trichkova T, Lenhardt M, Curtean-Bănăduc A (2016) The Lower Danube River-Danube Delta-North West Black Sea: A pivotal area of major interest for the past, present and future of its fish fauna - A short review. Sci Total Environ 545–546:137–151. https://doi.org/10.1016/j.scitotenv.2015.12.058
Ben Rais Lasram F, Guilhaumon F, Albouy C, Somot S, Thuiller W et al (2010) The Mediterranean Sea as a ‘cul-de-sac’ for endemic fishes facing climate change. Glob Chang Biol 16:3233–3245. https://doi.org/10.1111/j.1365-2486.2010.02224.x
Bin Othman AS, Goh GH, Todd PA (2010) The distribution and status of giant clams (family Tridacnidae)-a short review. Raffles Bull Zool 58:103–111
BirdLife International (2016a) Bulweria bifax. The IUCN red list of threatened species 2016:e.T22728804A94997177. https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22728804A94997177.en. Accessed 20 Oct 2022
BirdLife International (2016b) Camptorhynchus labradorius. The IUCN red list of threatened species 2016:e.T22680418A92862623. https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22680418A92862623.en. Accessed 20 Oct 2022
BirdLife International (2016c) Mergus australis. The IUCN red list of threatened species 2016:e.T22680496A92864737. https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22680496A92864737.en. Accessed 20 Oct 2022
BirdLife International (2016d) Pterodroma rupinarum. The IUCN red list of threatened species 2016:e.T22728800A94996980. https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22728800A94996980.en. Accessed 20 Oct 2022
BirdLife International (2016e) Phalacrocorax perspicillatus. The IUCN red list of threatened species 2016:e.T22696750A93584099. https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22696750A93584099.en. Accessed 20 Oct 2022
BirdLife International (2016f) Zapornia monasa. The IUCN red list of threatened species 2016:e.T22692708A93366211. https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22692708A93366211.en. Accessed 20 Oct 2022
BirdLife International (2017) Prosobonia cancellata (amended version of 2016 assessment). The IUCN red list of threatened species 2017:e.T62289108A119208101. https://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T62289108A119208101.en. Accessed 20 Oct 2022
BirdLife International (2021a) Haematopus meadewaldoi. The IUCN red list of threatened species 2021:e.T22693621A205917399. https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T22693621A205917399.en. Accessed 20 Oct 2022
BirdLife International (2021b) Pinguinus impennis. The IUCN red list of threatened species 2021:e.T22694856A205919631. https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T22694856A205919631.en. Accessed 20 Oct 2022
Bouchet P (1996a) Littoraria flammea. The IUCN red list of threatened species 1996:e.T12242A3329078. https://dx.doi.org/10.2305/IUCN.UK.1996.RLTS.T12242A3329078.en. Accessed 20 Oct 2022
Bouchet P (1996b) Lottia alveus. The IUCN red list of threatened species 1996:e.T12382A3339013. https://dx.doi.org/10.2305/IUCN.UK.1996.RLTS.T12382A3339013.en. Accessed 20 Oct 2022
Borja A, Elliott M, Basurko OC, Fernández Muerza A, Micheli F (2022) #Ocean Optimism: Balancing the Narrative About the Future of the Ocean. Front Mar Sci 9:886027. https://doi.org/10.3389/fmars.2022.886027
Brook A, Micco De V, Battipaglia G, Erbaggio A, Ludeno G et al (2020) A smart multiple spatial and temporal resolution system to support precision agriculture from satellite images: Proof of concept on Aglianico vineyard. Remote Sens Environ 240:111679. https://doi.org/10.1016/j.rse.2020.111679
Brown BE (1997) Coral bleaching: causes and consequences. Coral Reefs 16:S129–S138. https://doi.org/10.1007/s003380050249
Bryan GW, Gibbs PE, Hummerstone LG, Burt GR (1986) The Decline of the Gastropod Nucella Lapillus Around South-West England: Evidence for the Effect of Tributyltin from Antifouling Paints. J Mar Biolog Assoc 66:611–640. https://doi.org/10.1017/S0025315400042247
Cadée GC, Boon JP, Fischer CV, Mensink BP, Hallers-Tjabbes CCT (1995) Why the whelk (Buccinum undatum) has become extinct in the dutch Wadden Sea. Neth J Sea Res 34:i–ii. https://doi.org/10.1016/0077-7579(95)90044-6
Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C et al (2012) Biodiversity loss and its impact on humanity. Nature 486:59–67. https://doi.org/10.1038/nature11148
Carlton TJ (1993) Neoextinctions of Marine Invertebrates. Am Zool 33:499–509. https://doi.org/10.1093/icb/33.6.499
Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM et al (2015) Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci Adv 1(5):e1400253. https://doi.org/10.1126/sciadv.1400253
Chevaldonné P, Lejeusne C (2003) Regional warming-induced species shift in north-west Mediterranean marine caves. Ecol Lett 6:371–379. https://doi.org/10.1046/j.1461-0248.2003.00439.x
Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F et al (2010) The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS One 5:e11842. https://doi.org/10.1371/journal.pone.0011842
Constantinou CC, Georgiades GP, Loizou SG (2021) A Laser Vision System for Relative 3-D Posture Estimation of an Underwater Vehicle with Hemispherical Optics. Robot 10(4):126. https://doi.org/10.3390/robotics10040126
Cooley SD, Schoeman L, Bopp P, Boyd S, Donner DY et al (2022) Oceans and Coastal Ecosystems and Their Services. In: Pörtner HO, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K et al (eds) Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate. Cambridge University Press, Cambridge, UK and New York, NY, USA, pp 379–550. https://doi.org/10.1017/9781009325844.005
Cowie RH, Bouchet P, Fontaine B (2022) The Sixth Mass Extinction: fact, fiction or speculation? Biol Rev 97:640–663. https://doi.org/10.1111/brv.12816
Crocetta F, Bitar G, Zibrowius H, Oliverio M (2013) Biogeographical homogeneity in the eastern Mediterranean Sea. II. Temporal variation in Lebanese bivalve biota. Aquat Biol 19:75–84. https://doi.org/10.3354/ab00521
Davidson AD, Boyer AG, Kim H, Pompa-Mansilla S, Hamilton MJ et al (2012) Drivers and hotspots of extinction risk in marine mammals. Proc Natl Acad Sci U S A 109:3395–3400. https://doi.org/10.1073/pnas.1121469109
Descombes P, Wisz MS, Leprieur F, Parravicini V, Heine C et al (2015) Forecasted coral reef decline in marine biodiversity hotspots under climate change. Glob Chang Biol 21:2479–2487. https://doi.org/10.1111/gcb.12868
Dietzel A, Bode M, Connolly SR, Hughes TP (2021) The population sizes and global extinction risk of reef-building coral species at biogeographic scales. Nat Ecol Evol 5:663–669. https://doi.org/10.1038/s41559-021-01393-4
Domning D (2016) Hydrodamalis gigas. The IUCN red list of threatened species 2016:e.T10303A43792683. https://dx.doi.org/10.2305/IUCN.UK.2016-2.RLTS.T10303A43792683.en. Accessed 20 Oct 2022
Dong Y, Huang X, Reid DG (2015) Rediscovery of one of the very few ‘unequivocally extinct’ species of marine molluscs: Littoraria flammea (Philippi, 1847) lost, found—and lost again? J Molluscan Stud 81:313–321. https://doi.org/10.1093/mollus/eyv009
Dooley FD, Wyllie-Echeverria S, Roth MB, Ward PD (2013) Tolerance and response of Zostera marina seedlings to hydrogen sulfide. Aquat Bot 105:7–10. https://doi.org/10.1016/j.aquabot.2012.10.007
Duarte CM, Agusti S, Barbier E, Britten GL, Castilla JC et al (2020) Rebuilding marine life. Nature 580:39–51. https://doi.org/10.1038/s41586-020-2146-7
Dulvy N, Pinnegar J, Reynolds J (2009) Holocene extinctions in the sea, In: Turvey TS (ed) Holocene Extinctions. Oxford Academic, pp. 129–150. https://doi.org/10.1093/acprof:oso/9780199535095.003.0006
Dulvy NK, Davidson LNK, Kyne PM, Simpfendorfer CA, Harrison LR et al (2016) Ghosts of the coast: global extinction risk and conservation of sawfishes. Aquat Conserv 26:134–153. https://doi.org/10.1002/aqc.2525
Dulvy NK, Sadovy Y, Reynolds JD (2003) Extinction vulnerability in marine populations. Fish Fish 4:25–64. https://doi.org/10.1046/j.1467-2979.2003.00105.x
Dumbauld BR, Chapman JW, Torchin ME, Kuris AM (2011) Is the Collapse of Mud Shrimp (Upogebia pugettensis) Populations Along the Pacific Coast of North America Caused by Outbreaks of a Previously Unknown Bopyrid Isopod Parasite (Orthione griffenis)? Estuaries Coast 34:336–350. https://doi.org/10.1007/s12237-010-9316-z
Dutcher W (1891) The Labrador Duck: A Revised List of the Extant Specimens in North America, with Some Historical Notes. Auk 8(2):201–216. https://doi.org/10.2307/4068077
Essl F, Bacher S, Genovesi P, Hulme PE, Jeschke JM et al (2018) Which Taxa Are Alien? Criteria, Applications, and Uncertainties. Biosci 68:496–509. https://doi.org/10.1093/biosci/biy057
Estes JA, Burdin A, Doak DF (2016) Sea otters kelp forests and the extinction of Steller’s sea cow. Proc Natl Acad Sci USA 113:880–885. https://doi.org/10.1073/pnas.1502552112
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J et al (2011) Trophic downgrading of planet Earth. Science 333:301–306. https://doi.org/10.1126/science.1205106
Everett BI, Cliff G, Dudley SFJ, Wintner SP, van der Elst RP (2015) Do sawfish Pristis spp. represent South Africa’s first local extirpation of marine elasmobranchs in the modern era? Afr J Mar Sci 37:275–284. https://doi.org/10.2989/1814232X.2015.1027269
Florens FBV, Baider C (2007) Relocation of Omphalotropis plicosa (Pfeiffer, 1852), a Mauritian endemic landsnail believed extinct. J Molluscan Stud 73:205–206. https://doi.org/10.1093/mollus/eym004
Freeman LA, Kleypas JA, Miller AJ (2013) Coral Reef Habitat Response to Climate Change Scenarios. PLoS One 8:e82404. https://doi.org/10.1371/journal.pone.0082404
Freyhof J, Kottelat M (2008) Coregonus oxyrinchus. The IUCN red list of threatened species 2008: e.T5380A11126034. https://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T5380A11126034.en. Accessed 20 Oct 2022
Friess DA, Yando ES, Abuchahla GMO, Adams JB, Cannicci S et al (2020) Mangroves give cause for conservation optimism, for now. Curr Biol 30:R153–R154. https://doi.org/10.1016/j.cub.2019.12.054
Garrabou J, Gómez-Gras D, Medrano A, Cerrano C, Ponti M et al (2022) Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob Chang Biol 28:5708–5725. https://doi.org/10.1111/gcb.16301
Gazis I-Z, Greinert J (2021) Importance of Spatial Autocorrelation in Machine Learning Modeling of Polymetallic Nodules, Model Uncertainty and Transferability at Local Scale. Minerals 11:1172. https://doi.org/10.3390/min11111172
Gissi E, Manea E, Mazaris AD, Fraschetti S, Almpanidou V et al (2021) A review of the combined effects of climate change and other local human stressors on the marine environment. Sci Total Environ 755:142564. https://doi.org/10.1016/j.scitotenv.2020.142564
Giovos I, Kleitou P, Poursanidis D, Batjakas I, Bernardi G et al (2019) Citizen-science for monitoring marine invasions and stimulating public engagement: a case project from the eastern Mediterranean. Biol Invasions 21:3707–3721. https://doi.org/10.1007/s10530-019-02083-w
Glynn PW (1984) Widespread Coral Mortality and the 1982–83 El Niño Warming Event. Environ Conserv 11:133–146. https://doi.org/10.1017/S0376892900013825
Griffiths O (1996) Omphalotropis plicosa. The IUCN red list of threatened species 1996:e.T15297A4511585. https://dx.doi.org/10.2305/IUCN.UK.1996.RLTS.T15297A4511585.en. Accessed 20 Oct 2022
Gustafson RG, Waples RS, Myers JM, Weitkamp LA, Bryant GJ et al (2007) Pacific salmon extinctions: Quantifying lost and remaining diversity. Conserv Biol 21:1009–1020. https://doi.org/10.1111/j.1523-1739.2007.00693.x
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F et al (2008) A Global Map of Human Impact on Marine Ecosystems. Science 319:948–952. https://doi.org/10.1126/science.1149345
Hamilton SL, Saccomanno VR, Heady WN, Gehman AL, Lonhart SI, et al (2021) Disease-driven mass mortality event leads to widespread extirpation and variable recovery potential of a marine predator across the eastern Pacific. Proc R Soc B 288:20211195. https://doi.org/10.1098/rspb.2021.1195
Helgen K, Turvey ST (2016) Neovison macrodon. The IUCN red list of threatened species 2016:e.T40784A45204492. https://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T40784A45204492.en. Accessed 20 Oct 2022
Hilborn R, Amoroso RO, Anderson CM, Baum JK, Branch TA et al (2020) Effective fisheries management instrumental in improving fish stock status. Proc Natl Acad Sci U S A 117:2218–2224. https://doi.org/10.1073/pnas.1909726116
Hockey AP (1987) The Influence of Coastal Utilisation by Man on the Presumed Extinction of the Canarian Black Oystercatcher Haematopus meadewaldoi Bannerman. Biol Conserv 39:49–62. https://doi.org/10.1016/0006-3207(87)90006-1
Hongo C, Yamano H (2013) Species-Specific Responses of Corals to Bleaching Events on Anthropogenically Turbid Reefs on Okinawa Island, Japan, over a 15-year Period (1995-2009). PLoS One 8(4):e60952. https://doi.org/10.1371/journal.pone.0060952
Hortal J, Jiménez-Valverde A, Gómez JF, Lobo JM, Baselga A (2008) Historical bias in biodiversity inventories affects the observed environmental niche of the species. Oikos 117:847–858. https://doi.org/10.1111/j.0030-1299.2008.16434.x
Hughes TP, Bellwood DR, Connolly SR (2002) Biodiversity hotspots, centres of endemicity, and the conservation of coral reefs. Ecol Lett 5:775–784. https://doi.org/10.1046/j.1461-0248.2002.00383.x
Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A et al (2018) Global warming transforms coral reef assemblages. Nature 556:492–496. https://doi.org/10.1038/s41586-018-0041-2
IUCN (2022) The IUCN red list of threatened species. Version 2022-1. https://www.iucnredlist.org. Accessed 20 Oct 2022
Jacobsen MW, Hansen MM, Orlando L, Bekkevold D, Bernatchez L et al (2012) Mitogenome sequencing reveals shallow evolutionary histories and recent divergence time between morphologically and ecologically distinct European whitefish (Coregonus spp.). Mol Ecol 21:2727–2742. https://doi.org/10.1111/j.1365-294X.2012.05561.x
Katsanevakis S, Carella F, Çinar ME, Čižmek H, Jimenez C et al (2022) The Fan Mussel Pinna nobilis on the Brink of Extinction in the Mediterranean. In: DellaSala DA, Goldstein MI (eds) Imperilled: The Encyclopedia of Conservation. Elsevier, Oxford, pp 700–709. https://doi.org/10.1016/B978-0-12-821139-7.00070-2
Katsanevakis S, Coll M, Fraschetti S, Giakoumi S, Goldsborough D et al (2020) Twelve Recommendations for Advancing Marine Conservation in European and Contiguous Seas. Front Mar Sci 7. https://doi.org/10.3389/fmars.2020.565968
Katsanevakis S, Stelzenmüller V, South A, Sørensen TK, Jones PJS et al (2011) Ecosystem-based marine spatial management: Review of concepts, policies, tools, and critical issues. Ocean Coast Manag 54:807–820. https://doi.org/10.1016/j.ocecoaman.2011.09.002
Katsanevakis S, Wallentinus I, Zenetos A, Leppäkoski E, Çinar ME et al (2014) Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquat Invasions 9:391–423. https://doi.org/10.3391/ai.2014.9.4.01
Katsanevakis S, Weber A, Pipitone C, Leopold M, Cronin M et al (2012) Monitoring marine populations and communities: methods dealing with imperfect detectability. Aquat Biol 16:31–52. https://doi.org/10.3354/ab00426
Kayanne H, Hara T, Arai N, Yamano H, Matsuda H (2022) Trajectory to local extinction of an isolated dugong population near Okinawa Island, Japan. Sci Rep 12:6151. https://doi.org/10.1038/s41598-022-09992-2
Kriebel D, Tickner J, Epstein P, Lemons J, Levins R et al (2001) The precautionary principle in environmental science. Environ Health Perspect 109:871–876. https://doi.org/10.1289/ehp.01109871
Kroodsma DA, Mayorga J, Hochberg T, Miller NA, Boerder K et al (2018) Tracking the global footprint of fisheries. Science 359:904–908. https://doi.org/10.1126/science.aao5646
Krumhansl KA, Okamoto DK, Rassweiler A, Novak M, Bolton JJ et al (2016) Global patterns of kelp forest change over the past half-century. Proc Natl Acad Sci U S A 113:13785. https://doi.org/10.1073/pnas.1606102113
Lassalle G, Rochard E (2009) Impact of twenty-first century climate change on diadromous fish spread over Europe, North Africa and the Middle East. Glob Chang Biol 15:1072–1089. https://doi.org/10.1111/j.1365-2486.2008.01794.x
Lotze KH, Milewski I (2004) Two Centuries of Multiple Human Impacts and Successive Changes in a North Atlantic Food Web. Ecol Appl 14:1428–1447 http://www.jstor.org/stable/4493661
Lough JM (2012) Small change, big difference: Sea surface temperature distributions for tropical coral reef ecosystems, 1950–2011. J Geophys Res Oceans 117:C09018. https://doi.org/10.1029/2012JC008199
Lowry L (2015) Neomonachus tropicalis. The IUCN red list of threatened species 2015:e.T13655A45228171. https://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T13655A45228171.en. Accessed 20 Oct 2022
Lowry L (2017) Zalophus japonicus (amended version of 2015 assessmet). The IUCN red list of threatened species 2017:e.T41667A113089431. https://dx.doi.org/10.2305/IUCN.UK.2017-1.RLTS.T41667A113089431.en. Accessed 20 Oct 2022
Marques V, Castagné P, Fernández AP, Borrero-Pérez GH, Hocdé R et al (2021) Use of environmental DNA in assessment of fish functional and phylogenetic diversity. Conserv Biol 35:1944–1956. https://doi.org/10.1111/cobi.13802
Maslin M, Louis S, Godary Dejean K, Lapierre L, Villéger S et al (2021) Underwater robots provide similar fish biodiversity assessments as divers on coral reefs. Remote Sens Ecol Conserv 7:567–578. https://doi.org/10.1002/rse2.209
McAfee D, Doubleday ZA, Geiger N, Connell SD (2019) Everyone Loves a Success Story: Optimism Inspires Conservation Engagement. BioSci 69:274–281. https://doi.org/10.1093/biosci/biz019
McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH et al (2015) Marine defaunation: Animal loss in the global ocean. Sci 347:1255641. https://doi.org/10.1126/science.1255641
McClenachan L, Cooper BA (2008) Extinction rate, historical population structure and ecological role of the Caribbean monk seal. Proc R Soc B 275:1351–1358. https://doi.org/10.1098/rspb.2007.1757
Mehner T, Pohlmann K, Bittner D, Freyhof J (2018) Testing the devil’s impact on southern Baltic and North Sea basins whitefish (Coregonus spp.) diversity. BMC Ecol Biol 18:208. https://doi.org/10.1186/s12862-018-1339-2
Micheli F, Halpern BS, Walbridge S, Ciriaco S, Ferretti F et al (2013) Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLoS One 8:e79889. https://doi.org/10.1371/journal.pone.0079889
Millar AJK (2003) Vanvoorstia bennettiana (Royal Botanic Gardens Sydney, Australia). The IUCN red list of threatened species 2003:e.T43993A10838671. https://dx.doi.org/10.2305/IUCN.UK.2003.RLTS.T43993A10838671.en. Accessed 20 Oct 2022
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 339:b2535–b2535. https://doi.org/10.1136/bmj.b2535
Monte-Luna P, del Lluch-Belda D, Serviere-Zaragoza E, Carmona R, Reyes-Bonilla H et al (2007) Marine extinctions revisited. Fish Fish 8:107–122. https://doi.org/10.1111/j.1467-2679.2007.00240.x
Morais J, Medeiros APM, Santos BA (2018) Research gaps of coral ecology in a changing world. Mar Environ Res 140:243–250. https://doi.org/10.1016/j.marenvres.2018.06.021
Myers N (1993) Biodiversity and the Precautionary Principle. Ambio 22:74–79
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315:1846–1850. https://doi.org/10.1126/science.1138657
Nehru P, Balasubramanian P (2018) Mangrove species diversity and composition in the successional habitats of Nicobar Islands, India: A post-tsunami and subsidence scenario. For Ecol Manag 427:70–77. https://doi.org/10.1016/j.foreco.2018.05.063
Neo ML, Wabnitz CC, Braley DR, Heslinga AG, Fauvelot C et al (2017) Giant Clams (Bivalvia: Cardiidae: Tridacninae): A Comprehensive Update of Species and their Distribution, Current Threats and Conservation Status. In: Hawkins SJ, Evans AJ, Dale L, Firth DJ, Hughes IP, Smith IP (eds) Oceanography and Marine Biology. CRC Press, Boca Raton, p 498. https://doi.org/10.1201/b21944
O’Dea RE, Lagisz M, Jennions MD, Koricheva J, Noble DWA et al (2021) Preferred reporting items for systematic reviews and meta-analyses in ecology and evolutionary biology: a PRISMA extension. Biol Rev 96:1695–1722. https://doi.org/10.1111/brv.12721
O’Hara CC, Frazier M, Halpern BS (2021) At-risk marine biodiversity faces extensive, expanding, and intensifying human impacts. Sci 372:84–87. https://doi.org/10.1126/science.abe6731
Olson SL (1975) Paleornithology of St. Helena Island, South Atlantic Ocean. Smithson Contrib Paleobiol Number 23. https://doi.org/10.5479/si.00810266.23.1
Pecl GT, Araújo MB, Bell JD, Blanchard J, Bonebrake TC et al (2017) Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355:eaai9214. https://doi.org/10.1126/science.aai9214
Peukert A, Petersen S, Greinert J, Charlot F (2018) Seabed Mining. In: Micallef A, Krastel S, Savini A (eds) Submarine Geomorphology. Springer Geology, pp 481–502. https://doi.org/10.1007/978-3-319-57852-1_24
Phillips JA, Blackshaw JK (2011) Extirpation of Macroalgae (Sargassum spp.) on the Subtropical East Australian Coast. Conserv Biol 25:913–921. https://doi.org/10.1111/j.1523-1739.2011.01727.x
Pikitch EK, Doukakis P, Lauck L, Chakrabarty P, Erickson DL (2005) Status, trends and management of sturgeon and paddlefish fisheries. Fish Fish 6:233–265. https://doi.org/10.1111/j.1467-2979.2005.00190.x
Pisano A, Marullo S, Artale V, Falcini F, Yang C et al (2020) New Evidence of Mediterranean Climate Change and Variability from Sea Surface Temperature Observations. Remote Sens 12:132. https://doi.org/10.3390/rs12010132
Poquita-Du RC, Quek ZBR, Jain SS, Schmidt-Roach S, Tun K et al (2019) Last species standing: loss of Pocilloporidae corals associated with coastal urbanization in a tropical city state. Mar Biodivers 49:1727–1741. https://doi.org/10.1007/s12526-019-00939-x
Powell CL (2022) The extinct limpet Lottia edmitchelli (Lipps, 1963) from the Southern California Bight, USA. PaleoBios 39:1–7. https://doi.org/10.5070/P939357897
Qiwei W (2022) Psephurus gladius. The IUCN red list of threatened species 2022:e.T18428A146104283. https://dx.doi.org/10.2305/IUCN.UK.2022-1.RLTS.T18428A146104283.en. Accessed 20 Jan 2023
Rasher DB, Steneck RS, Halfar J, Kroeker KJ, Ries JB et al (2020) Keystone predators govern the pathway and pace of climate impacts in a subarctic marine ecosystem. Sci 369:1351–1355. https://doi.org/10.1126/SCIENCE.AAV7515
Rawlence NJ, Till CE, Easton LJ, Spencer HG, Schuckard R et al (2017) Speciation, range contraction and extinction in the endemic New Zealand King Shag complex. Mol Phylogenet Evol 115:197–209. https://doi.org/10.1016/j.ympev.2017.07.011
Richards ZT, Day JC (2018) Biodiversity of the Great Barrier Reef—how adequately is it protected? Peer J 6:e4747. https://doi.org/10.7717/peerj.4747
Richards ZT, Juszkiewicz DJ, Hoggett A (2021) Spatio-temporal persistence of scleractinian coral species at Lizard Island, Great Barrier Reef. Coral Reefs 40:1369–1378. https://doi.org/10.1007/s00338-021-02144-4
Rilov G (2016) Multi-species collapses at the warm edge of a warming sea. Sci Rep 6:36897. https://doi.org/10.1038/srep36897
Rilov G, Fraschetti S, Gissi E, Pipitone C, Badalamenti F et al (2020) A fast-moving target: achieving marine conservation goals under shifting climate and policies. Ecol Appl 30:e02009. https://doi.org/10.1002/eap.2009
Rilov G, Mazaris A, Stelzenmüller V, Helmuth B, Wahl M et al (2019) Adaptive marine conservation planning in the face of climate change: What can we learn from physiological, genetic and ecological studies? Glob Ecol Conserv 17:e00566. https://doi.org/10.1016/j.gecco.2019.e00566
Roman L, Schuyler Q, Wilcox C, Hardesty BD (2021) Plastic pollution is killing marine megafauna, but how do we prioritize policies to reduce mortality? Conserv Lett 14:e12781. https://doi.org/10.1111/conl.12781
Rounsevell MDA, Harfoot M, Harrison PA, Newbold T, Gregory RD et al (2020) A biodiversity target based on species extinctions. Science 368:1193–1195. https://doi.org/10.1126/science.aba6592
RStudio Team (2022) RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA URL: http://www.rstudio.com/
Smale DA, Wernberg T (2013) Extreme climatic event drives range contraction of a habitat-forming species. Proc R Soc B 280:20122829. https://doi.org/10.1098/rspb.2012.2829
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA et al (2007) Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioSci 57:573–583. https://doi.org/10.1641/B570707
Springer AM, Estes JA, van Vliet GB, Williams TM, Doak DF et al (2003) Sequential megafaunal collapse in the North Pacific Ocean: An ongoing legacy of industrial whaling? Proc Natl Acad Sci USA 100:12223–12228. https://doi.org/10.1073/pnas.1635156100
Stauffer S, Jucker M, Keggin T, Marques V, Andrello M et al (2021) How many replicates to accurately estimate fish biodiversity using environmental DNA on coral reefs? Ecol Evol 11:14630–14643. https://doi.org/10.1002/ece3.8150
Stefanni S, Mirimin L, Stanković D, Chatzievangelou D, Bongiorni L et al (2022) Framing Cutting-Edge Integrative Deep-Sea Biodiversity Monitoring via Environmental DNA and Optoacoustic Augmented Infrastructures. Front Mar Sci 8:2296–7745. https://doi.org/10.3389/fmars.2021.797140
Stelfox M, Hudgins J, Sweet M (2016) A review of ghost gear entanglement amongst marine mammals, reptiles and elasmobranchs. Mar Pollut Bull 111:6–17. https://doi.org/10.1016/j.marpolbul.2016.06.034
Stelzenmüller V, Coll M, Cormier R, Mazaris AD, Pascual M et al (2020) Operationalizing risk-based cumulative effect assessments in the marine environment. Sci Total Environ 724:138118. https://doi.org/10.1016/j.scitotenv.2020.138118
Stelzenmüller V, Coll M, Mazaris AD, Giakoumi S, Katsanevakis S et al (2018) A risk-based approach to cumulative effect assessments for marine management. Sci Total Environ 612:1132–1140. https://doi.org/10.1016/j.scitotenv.2017.08.289
Stuart-Smith DR, Edgar JG, Barret SN, Bets EA, Baker CS et al (2017) Assessing National Biodiversity Trends for Rocky and Coral Reefs through the Integration of Citizen Science and Scientific Monitoring Programs. BioSci 67(2):134–146. https://doi.org/10.1093/biosci/biw180
Tsirintanis K, Azzurro E, Crocetta F, Dimiza M, Froglia C et al (2022) Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat Invasions 17:308–352. https://doi.org/10.3391/ai.2022.17.3.01
Turvey ST, Risley CL (2006) Modelling the extinction of Steller’s sea cow. Biol Lett 2:94–97. https://doi.org/10.1098/rsbl.2005.0415
Unsworth RKF, McKenzie LJ, Collier CJ, Cullen-Unsworth LC, Duarte CM et al (2019) Global challenges for seagrass conservation. Ambio 48:801–815. https://doi.org/10.1007/s13280-018-1115-y
van der Meij SET, Moolenbeek RG, Hoeksema BW (2009) Decline of the Jakarta Bay molluscan fauna linked to human impact. Mar Pollut Bull 59:101–107. https://doi.org/10.1016/j.marpolbul.2009.02.021
van Katwijk MM, Bos AR, Kennis P, de Vries R (2010) Vulnerability to eutrophication of a semi-annual life history: A lesson learnt from an extinct eelgrass (Zostera marina) population. Biol Conserv 143:248–254. https://doi.org/10.1016/j.biocon.2009.08.014
Valiente-Banuet A, Aizen MA, Alcántara JM, Arroyo J, Cocucci A et al (2015) Beyond species loss: the extinction of ecological interactions in a changing world. Funct Ecol 29:299–307. https://doi.org/10.1111/1365-2435.12356
Valentini A, Taberlet P, Miaud C, Civade R, Herder J et al (2016) Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol Ecol 25:929–942. https://doi.org/10.1111/mec.13428
Verhelst P, Reubens J, Buysse D, Goethals P, van Wichelen J et al (2021) Toward a roadmap for diadromous fish conservation: the Big Five considerations. Front Ecol Environ 19:396–403. https://doi.org/10.1002/fee.2361
Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison W et al (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci U S A 106:12377–12381. https://doi.org/10.1073/pnas.0905620106
Wernberg T, Bennett S, Babcock RC, De Bettignies T, Cure K et al (2016) Climate-driven regime shift of a temperate marine ecosystem. Science 353:169–172. https://doi.org/10.1126/science.aad8745
West D, David B, Ling N (2014) Prototroctes oxyrhynchus. The IUCN Red List of Threatened Species 2014:e.T18384A20887241. https://dx.doi.org/10.2305/IUCN.UK.2014-3.RLTS.T18384A20887241.en. Accessed 20 Oct 2022
Worm B, Tittensor DP (2011) Range contraction in large pelagic predators. Proc Natl Acad Sci U S A 108:11942–11947. https://doi.org/10.1073/pnas.1102353108
Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C et al (2006) Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science 314:787–790. https://doi.org/10.1126/science.1132294
Yan HF, Kyne PM, Jabado RW, Leeney RH, Davidson LNK (2021) Overfishing and habitat loss drives range contraction of iconic marine fishes to near extinction. Sci Adv 7:eabb6026. https://doi.org/10.1126/sciadv.abb6026
Yeruham E, Rilov G, Shigel M, Abelson A (2015) Collapse of the echinoid Paracentrotus lividus populations in the Eastern Mediterranean—result of climate change? Sci Rep 5(1):13479
Yeruham E, Shpigel M, Abelson A, Rilov G (2020) Ocean warming and tropical invaders erode the performance of a key herbivore. Ecology 101:e02925. https://doi.org/10.1002/ecy.2925
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
Conceptualization: AN, SK; Methodology: AN, SK; Formal analysis and investigation: AN, SK; Writing - original draft preparation: AN; Writing – review and editing: SK; Screening at all stages and data collection; AN; Supervision: SK
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest
Additional information
Communicated by Dror Angel
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nikolaou, A., Katsanevakis, S. Marine extinctions and their drivers. Reg Environ Change 23, 88 (2023). https://doi.org/10.1007/s10113-023-02081-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10113-023-02081-8