Abstract
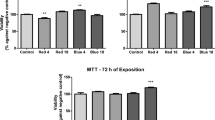
Photobiomodulation-based (PBM-based) therapies show promising results in mucositis and dermatitis treatment by stimulating wound healing mechanisms such as cell proliferation and migration. The aim of the present study is to investigate the in vitro effects of CareMin650 on the proliferation and migration of two different types of cells, namely cancer and non-cancer cells, with or without X-ray radiation. Study design used PBM through a combination of 0-3-6 J/cm2 doses—with or without X-ray radiation—on the proliferation and migration capabilities of a keratinocyte cell line (HaCaT) and a squamous cell carcinoma line (SCC61). PBM is delivered by a new woven optical fiber device, namely CareMin650 prototype (light emission by LEDs (light-emitting diodes), peak at 660 nm, irradiance of 21.6 mW/cm2). The effectiveness of PBM to increase HaCaT proliferation and migration (with or without X-ray radiation) supports the capability of PBM to favor wound healing. It also highlights that PBM does not provide any anti-radiation effect to previously X-rays radiated SCC (p < 0.001). Such data supports the beneficial effect of PBM delivered by an optical fiber device to heal wounds, without promoting cancer development.



Similar content being viewed by others
Data availability
HaCaT is a spontaneously transformed keratinocyte cell line from adult human skin. SCC is a head and neck squamous cell carcinoma (HNSCC) cell line, derived from a tongue cancer. These cell lines were obtained from the American Type Culture Collection (ATCC, USA).
References
Singh M, Alavi A, Wong R, Akita S (2016) Radiodermatitis: a review of our current understanding. Am J Clin Dermatol 17:277–292. https://doi.org/10.1007/s40257-016-0186-4
Maria OM, Eliopoulos N, Muanza T (2017) Radiation-induced oral mucositis. Front Oncol 7. https://doi.org/10.3389/fonc.2017.00089
Sonis ST, Elting LS, Keefe D et al (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100:1995–2025. https://doi.org/10.1002/cncr.20162
Zecha JAEM, Raber-Durlacher JE, Nair RG et al (2016) Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 24:2781–2792. https://doi.org/10.1007/s00520-016-3152-z
Bjordal JM, Bensadoun R-J, Tunèr J et al (2011) A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support Care Cancer 19:1069–1077. https://doi.org/10.1007/s00520-011-1202-0
Migliorati C, Hewson I, Lalla RV et al (2013) Systematic review of laser and other light therapy for the management of oral mucositis in cancer patients. Support Care Cancer 21:333–341. https://doi.org/10.1007/s00520-012-1605-6
Bensadoun R-J, Nair R (2012) Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Curr Opin Oncol 24:363–370. https://doi.org/10.1097/CCO.0b013e328352eaa3
DeLand MM, Weiss RA, McDaniel DH, Geronemus RG (2007) Treatment of radiation-induced dermatitis with light-emitting diode (LED) photomodulation. Lasers Surg Med 39:164–168. https://doi.org/10.1002/lsm.20455
Censabella S, Claes S, Robijns J et al (2016) Photobiomodulation for the management of radiation dermatitis: the DERMIS trial, a pilot study of MLS® laser therapy in breast cancer patients. Support Care Cancer 24:3925–3933. https://doi.org/10.1007/s00520-016-3232-0
Zadik Y, Arany PR, Fregnani ER et al (2019) Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 27:3969–3983. https://doi.org/10.1007/s00520-019-04890-2
Robijns J, Lodewijckx J, Mebis J (2019) Photobiomodulation therapy for acute radiodermatitis. Curr Opin Oncol 31:291–298. https://doi.org/10.1097/CCO.0000000000000511
Jenkins P, Carroll J (2011) How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg 29:785–787. https://doi.org/10.1089/pho.2011.9895
Tunér J, Jenkins PA (2016) Parameter reproducibility in photobiomodulation. Photomed Laser Surg 34:91–92. https://doi.org/10.1089/pho.2016.4105
Lalla RV, Bowen J, Barasch A et al (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy: MASCC/ISOO Mucositis Guidelines. Cancer 120:1453–1461. https://doi.org/10.1002/cncr.28592
Huang Y-Y, Sharma SK, Carroll J, Hamblin MR (2011) Biphasic dose response in low level light therapy-an update. Dose-Response Publ Int Hormesis Soc 9:602–618. https://doi.org/10.2203/dose-response.11-009.Hamblin
Guy J-B, Espenel S, Vallard A et al (2017) Evaluation of the cell invasion and migration process: a comparison of the video microscope-based scratch wound assay and the Boyden chamber assay. J Vis Exp JoVE. https://doi.org/10.3791/56337
Liao X, Xie G-H, Liu H-W et al (2014) Helium-neon laser irradiation promotes the proliferation and migration of human epidermal stem cells in vitro: proposed mechanism for enhanced wound re-epithelialization. Photomed Laser Surg 32:219–225. https://doi.org/10.1089/pho.2013.3667
Hamblin MR, Nelson ST, Strahan JR (2018) Photobiomodulation and cancer: what is the truth? Photomed Laser Surg 36:241–245. https://doi.org/10.1089/pho.2017.4401
Gagnon D, Gibson TWG, Singh A et al (2016) An in vitro method to test the safety and efficacy of low-level laser therapy (LLLT) in the healing of a canine skin model. BMC Vet Res 12:73. https://doi.org/10.1186/s12917-016-0689-5
Ramos Silva C, Cabral FV, de Camargo CFM et al (2016) Exploring the effects of low-level laser therapy on fibroblasts and tumor cells following gamma radiation exposure. J Biophotonics 9:1157–1166. https://doi.org/10.1002/jbio.201600107
Teuschl A, Rosado balmayor E, Redl H et al (2015) Phototherapy with LED light modulates healing processes in an in vitro scratch-wound model using 3 different cell types. Dermatol Surg 41:261–268
Fushimi T, Inui S, Nakajima T et al (2012) Green light emitting diodes accelerate wound healing: characterization of the effect and its molecular basis in vitro and in vivo. Wound Repair Regen 20:226–235. https://doi.org/10.1111/j.1524-475X.2012.00771.x
Barasch A, Raber-Durlacher J, Epstein JB, Carroll J (2016) Effects of pre-radiation exposure to LLLT of normal and malignant cells. Support Care Cancer 24:2497–2501. https://doi.org/10.1007/s00520-015-3051-8
Kim K, Lee J, Jang H et al (2019) Photobiomodulation enhances the angiogenic effect of mesenchymal stem cells to mitigate radiation-induced enteropathy. Int J Mol Sci 20. https://doi.org/10.3390/ijms20051131
Pastore D, Greco M, Passarella S (2000) Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol 76:863–870. https://doi.org/10.1080/09553000050029020
Hamblin MR, Agrawal T, de Sousa M (2016) Chapter 10 Molecular Basis for photobiomodulation: light-induced nitric oxide synthesis by cytochrome c oxidase in low-level laser therapy. In: Handbook of low-level laser therapy. CRC Press, Boca Raton
Hamblin MR, Agrawal T, de Sousa M (2016) Chapter 16 Low-level laser (light) therapy for wound healing in animal models. In: Handbook of low-level laser therapy. CRC Press, Boca Raton
Day RM, Suzuki YJ (2006) Cell proliferation, reactive oxygen and cellular glutathione. Dose-Response 3:425–442. https://doi.org/10.2203/dose-response.003.03.010
Hurd TR, DeGennaro M, Lehmann R (2012) Redox regulation of cell migration and adhesion. Trends Cell Biol 22:107–115. https://doi.org/10.1016/j.tcb.2011.11.002
Hamblin MR, Agrawal T, de Sousa M (2016) Chapter 9 Role of reactive oxygen species in low-level laser therapy. In: Handbook of low-level laser therapy. CRC Press, Boca Raton
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron Publ IEEE Lasers Electro-Opt Soc 22. https://doi.org/10.1109/JSTQE.2016.2561201
Karu T, Pyatibrat L, Kalendo G (1994) Irradiation with He--Ne laser can influence the cytotoxic response of HeLa cells to ionizing radiation. Int J Radiat Biol 65:691–697. https://doi.org/10.1080/09553009414550811
Cuiffo BG, Karnoub AE (2012) Mesenchymal stem cells in tumor development. Cell Adhes Migr 6:220–230. https://doi.org/10.4161/cam.20875
Franken NAP, Oei AL, Kok HP et al (2013) Cell survival and radiosensitisation: modulation of the linear and quadratic parameters of the LQ model (review). Int J Oncol 42:1501–1515. https://doi.org/10.3892/ijo.2013.1857
Acknowledgments
This work was conducted at the Faculty of Medicine Lyon Sud of Lyon 1 University (UMS2444/US8).
Author information
Authors and Affiliations
Contributions
Conceptualization: Fabrice Axisa, Elodie Courtois, Laure Alston, Pierre Saint-Girons; methodology: Jean-Baptiste Guy, Elodie Courtois, René-Jean Bensadoun; formal analysis and investigation: Elodie Courtois, Jean-Baptiste Guy, Narimène Houmera; writing–original draft preparation: Elodie Courtois; writing–review and editing: Fabrice Axisa, Nicolas Magné, Anne Visbecq; resources: full name; supervision: Claire Rodriguez-Lafrasse, Pierre Saint-Girons.
Corresponding author
Ethics declarations
Conflict of interest
Elodie Courtois (R&D Engineer), Fabrice Axisa (R&D Director), Pierre Saint-Girons (CEO), and Anne Visbecq (CMO) are employees of NeoMedLight. The authors declare that they have no other conflict of interest. The authors had full control of the primary data and agree to allow review of said data, if requested by the journal.
Code availability
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Courtois, E., Guy, JB., Axisa, F. et al. Photobiomodulation by a new optical fiber device: analysis of the in vitro impact on proliferation/migration of keratinocytes and squamous cell carcinomas cells stressed by X-rays. Lasers Med Sci 36, 1445–1454 (2021). https://doi.org/10.1007/s10103-020-03185-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03185-x