Abstract
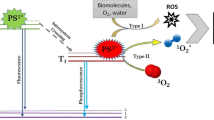
The use of eosin methylene blue according to Giemsa as photosensitizer is presented for the first time in this paper. The present study evaluated the potential application of chlorophyllin sodium copper salt (CuChlNa) and eosin methylene blue according to Giemsa (EMB) as antimicrobial photosensitizers (aPS) for photodynamic inactivation (PDI) of Staphylococcus aureus (gram-positive) and Escherichia coli (gram-negative) bacteria. The experiments were performed using S. aureus stain ATCC 25923 and E. coli ATCC 25922 in which five aPS concentrations (0.0, 1.0, 2.5, 5.0, 10.0, and 20.0 μM for S. aureus and 0.0, 5.0, 10.0, 20.0, 40.0, and 50.0 μM for E. coli) were prepared and added in 2 mL of a saline solution containing the bacterial inoculum. After aPS incubation, the samples were divided into two groups, one kept in the dark and another submitted to the illumination. Then, the bacterial inactivation was determined 18 h after the incubation at 37 °C by counting the colony-forming units (CFU). The results revealed that both EMB and CuChlNa can be used as aPS for the photoinactivation of S. aureus, while only EMB was able to photoinactivate E. coli. Nevertheless, a more complex experimental setup was needed for photoinactivation of E. coli. The data showed that EMB and CuChlNa presented similar photoinactivation effects on S. aureus, in which bacterial growth was completely inhibited at photosensitizer (PS) concentrations over 5 μM, when samples were previously incubated for 30 min and irradiated by a light dose of 30 J cm−2 as a result of an illumination of 1 h at 8.3 mW cm−2 by using a red light at 625 nm with a 1 cm beam diameter and output power of 6.5 mW. In the case of E. coli, bacterial growth was completely inhibited only when combining a PS incubation period of 120 min with concentrations over 20 μM.




Similar content being viewed by others
References
Runcie H (2015) Infection in a pre-antibiotic era. J Anc Dis Prev Rem 3:125
Manzetti S, Ghisi R (2014) The environmental release and fate of antibiotics. Mar Pollut Bull 79:7–15
Arias CA, Murray BE (2009) Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med 360:439–443
Alanis AJ (2005) Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res 36:697–705
Sperandio FF, Huang Y, Hamblin MR (2013) Antimicrobial photodynamic therapy to kill gram-negative bacteria. Recent Pat Antiinfect Drug Discov 8:108–120
Salton MRJ, Kim KS (1996) Structure. in: Baron S (ed) medical microbiology, 4rd edn. Galveston, University of Texas Medical Branch at Galveston. pp 1–20. http://www.ncbi.nlm.nih.gov/books/NBK8477/. Accessed 21 august 2016
Foster T (1996) Staphylococcus. in: Baron S (ed) medical microbiology, 4rd edn. Galveston, University of Texas Medical Branch at Galveston. pp 1–22. http://www.ncbi.nlm.nih.gov/books/NBK8448/. Accessed 21 august 2016
Guentzel MN (1996) Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus. In: Baron S. (ed) medical microbiology, 4rd edn. Galveston, University of Texas Medical Branch at Galveston, pp 1–20. http://www.ncbi.nlm.nih.gov/books/NBK8035/. Accessed 21 august 2016
Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, QAMAR FN, Mir F, Kariuki S, Bhutta ZQA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O (2013) Antibiotic resistance—the need for global solutions. Lancet Infect Dis 13:1057–1098
Centers for Disease Control and Prevention (2013) Antibiotic resistance threats in the United States. http: //www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed 21 august 2016
Liu Y, Wang Y, Walsh TR, Yi L, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J, Shen J (2016) Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168
Foote CS (1991) Definition of type I and type II photosensitized oxidation. Photochem Photobiol 54:659
Henderson BW, Doughert TJ (1992) How does photodynamic therapy work? Photochem Photobiol 55:145–157
Vera DM, Haynes MH, Ball AR, Dai T, Astrakas C, Kelso MJ, Hamblin MR, Tegos GP (2012) Strategies to potentiate antimicrobial photoinactivation by overcoming resistant phenotypes. Photochem Photobiol 88:499–511
Hamblin MR (2016) Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes. Curr Opin Microbiol 33:67–73
Bumah VV, Whelan HT, Masson-Meyers DN, Quirk B, Buchmann E, Enwemeka CS (2015) The bactericidal effect of 470-nm light and hyperbaric oxygen on methicillin-resistant Staphylococcus aureus (MRSA). Lasers Med Sci 30:1153–1159
Dai T (2017) The antimicrobial effect of blue light: what are behind? Virulence DOI. doi:10.1080/21505594.2016.1276691
Enwemeka CS (2013) Antimicrobial blue light: an emerging alternative to antibiotics. Photomed Laser Surg 31:509–511
Barcia JJ (2007) The Giemsa stain: its history and applications. Int J Surg Pathol 15:292–296
Brammer SP, Toniazzo C, Poersch LB (2015) Corantes comumente empregados na citogenética vegetal. Arq Inst Biol 82:1–8
Fleischer B (2004) 100 years ago: Giemsa’s solution for staining of plasmodia. Tropical Med Int Health 9:755–756
Gerola AP, Santana A, França PB, Tsubone TM, Oliveira HPM, Caetano W, Kimura E, Hioka N (2011) Effects of metal and the phytyl chain on chlorophyll derivatives: physicochemical evaluation for photodynamic inactivation of microorganisms. Photochem Photobiol 87:884–894
Park JH, Moon YH, Bang IS, Kim YC, Kim SA, Ahn SG, Yoon JH (2010) Antimicrobial effect of photodynamic therapy using a highly pure chlorin e6. Lasers Med Sci 25:705–710
Sahu K, Sharma M, Bansal H, Dube A, Gupt PK (2013) Topical photodynamic treatment with poly-L-lysine–chlorin p6 conjugate improves wound healing by reducing hyperinflammatory response in Pseudomonas aeruginosa-infected wounds of mice. Lasers Med Sci 28:465–471
Luksiene Z, Buchovec I, Paskeviciute E (2010) Inactivation of Bacillus cereus by Na-chlorophyllin-based photosensitization on the surface of packaging. J Appl Microbiol 109:1540–1548
Freshney IR (2005) Culture of animal cells, a manual of basic technique, 5th edn. Wiley-Liss, New York
Skehan P, Storeng R, Scudiero D, Monks A, Mcmahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langlet J, Cronise P, Vaigro-Wolff A, Ray GM, Campbell H, Mayo J, Boyd M (1991) Feasibility of a high-flux anticancer drug screen using diverse panel of cultured human tumor cell lines. Natl Cancer Inst 83:757–766
Shoemaker RH (2006) The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6:813–823
Durantini EN (2006) Photodynamic inactivation of bacteria. Curr Bioact Compd 2:127–142
Nitzan Y, Gutterman M, Malik Z, Ehrenberg B (1992) Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol 55:89–96
Tang HM, Hamblin MR, Yow CMN (2007) A comparative in vitro photoinactivation study of clinical isolates of multidrug-resistant pathogens. J Infect Chemother 13:87–91
Huang L, Dai T, Hamblin MR (2010) Antimicrobial photodynamic inactivation and photodynamic therapy for infections. Methods Mol Biol 635:155–173
López-Carballo G, Hernández-Muñoz P, Gavara R, Ocio MJ (2008) Photoactivated chlorophyllin-based gelatin films and coatings to prevent microbial contamination of food products. Int J Food Microbiol 126:65–70
Acknowledgments
The authors acknowledge the technical support provided by Mr. Esmael Dias Prado and Marcelo Alves Teixeira.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by the National Institute of Science and Technology of Optics and Photonics (Grant 440585/2016-3).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures were performed according to the ethical standards of the Federal University of Mato Grosso do Sul. An ethical approval was not needed.
Informed consent
Informed consent was not needed.
Electronic supplementary material
ESM. 1
(DOCX 2682 kb)
Rights and permissions
About this article
Cite this article
Caires, C.S.A., Leal, C.R.B., Ramos, C.A.N. et al. Photoinactivation effect of eosin methylene blue and chlorophyllin sodium-copper against Staphylococcus aureus and Escherichia coli . Lasers Med Sci 32, 1081–1088 (2017). https://doi.org/10.1007/s10103-017-2210-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2210-1