Abstract
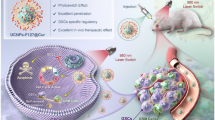
Surface plasmon resonance effect of gold nanostructures makes them good candidates for photothermal therapy (PTT) application. Herein, gold-ferrite nanocomposite (GFNC) was synthesized and characterized as a photothermal agent in PTT. The aim of this study was to investigate the effect of GFNC upon laser irradiation on treatment of cancer in mice bearing melanoma cancer. Thirty mice received 1.5 × 106 B16/F10 cells subcutaneously. After 1 week, the mice bearing solid tumor were divided into four groups: control group (without any treatment), laser group (received laser irradiation without GFNC injection), GFNC group (only received intratumorally GFNC), and GFNC + laser group (received intratumorally GFNC upon laser irradiation). In GFNC + laser group, 200 μL of fluid, 1.3 × 10−7 mol L−1 gold nanoparticles, was injected intratumorally and immediately the site of tumor was exposed to continuous wave diode laser beam (808 nm, 1.6 W cm−2) for 15 min. All mice but four were euthanized 24 h after treatment to compare the necrotic surface area histologically by using measuring graticule. Statistical analyses revealed significant differences in necrosis extent for GFNC + laser group, compared to other groups. Four subjects (control group and GFNC + laser group, two mice each) were kept for longitudinal study. Histological analyses and tumor volume measurements of the four subjects indicated that tumor in GFNC + laser group was controlled appropriately. It was concluded that combining an 808-nm laser at a power density of 1.6 W cm−2 with GFNC has a destruction effect in melanoma cancer cells in an animal model.





Similar content being viewed by others
References
Ji Z, Lin G, Lu Q et al (2012) Targeted therapy of SMMC-7721 liver cancer in vitro and in vivo with carbon nanotubes based drug delivery system. J Colloid Interface Sci 365(1):143–149. doi:10.1016/j.jcis.2011.09.013
Alexis F, Rhee JW, Richie JP et al (2008) New frontiers in nanotechnology for cancer treatment. Urol Oncol 26(1):74–85. doi:10.1016/j.urolonc.2007.03.017
Betrouni N, Colin P, Bozzini G et al (2013) Image-guided laser therapies for prostate cancer. IRBM 34:28–32. doi:10.1016/j.irbm.2012.12.006
Huang X, El-Sayed MA (2011) Plasmonic photo-thermal therapy (PPTT). Alex J Med 47:1–9. doi:10.1016/j.ajme.2011.01.001
Master A, Livingston M, Sen Gupta A (2013) Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges. J Control Release 168(1):88–102. doi:10.1016/j.jconrel.2013.02.020
Weissleder R (2001) A clearer vision for in vivo imaging. Nat Biotechnol 19(4):316–317
Colin P, Nevoux P, Marqa M et al (2012) Focal laser interstitial thermotherapy (LITT) at 980 nm for prostate cancer: treatment feasibility in Dunning R3327-AT2 rat prostate tumour. BJU Int 109(3):452–458. doi:10.1111/j.1464-410X.2011.10406.x
van Nimwegen SA, L’Eplattenier HF, Rem AI et al (2009) Nd:YAG surgical laser effects in canine prostate tissue: temperature and damage distribution. Phys Med Biol 54(1):29–44. doi:10.1088/0031-9155/54/1/003
Sassaroli E, Li KCP, O’Neill BE (2009) Numerical investigation of heating of a gold nanoparticle and the surrounding microenvironment by nanosecond laser pulses for nanomedicine applications. Phys Med Biol 54:5541–5560. doi:10.1088/0031-9155/54/18/013
Huang X, Jain P, El-Sayed I et al (2008) Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 23:217–228. doi:10.1007/s10103-007-0470-x
O’Neal DP, Hirsch LR, Halas NJ et al (2004) Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett 209:171–176
Terentyuk GS, Maslyakova GN, Suleymanova LV et al (2009) Laser-induced tissue hyperthermia mediated by gold nanoparticles: toward cancer phototherapy. J Biomed Opt 14:021016. doi:10.1117/1.3122371
Xiao Y, Gao X, Taratula O et al (2009) Anti-HER2 IgY antibody-functionalized single-walled carbon nanotubes for detection and selective destruction of breast cancer cells. BMC Cancer 9:351. doi:10.1186/1471-2407-9-351
Zhou F, Xing D, Ou Z et al (2009) Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J Biomed Opt 14:021009. doi:10.1117/1.3078803
Cheng Y, Meyers JD, Broome AM et al (2011) Deep penetration of a PDT drug into tumors by noncovalent drug-gold nanoparticle conjugates. J Am Chem Soc 133:2583–2591
Zeng S, Yong KT, Roy I (2011) A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 6:491–506. doi:10.1007/s11468-011-9228-1
Bardhan R, Lal S, Joshi A et al (2011) Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res 44(10):936–946. doi:10.1021/ar200023x
Khlebtsov N, Bogatyrev V, Dykman L et al (2013) Analytical and theranostic applications of gold nanoparticles and multifunctional nanocomposites. Theranostics 3(3):167–180. doi:10.7150/thno.5716
Khlebtsov B, Panfilova E, Khanadeev V et al (2011) Nanocomposites containing silica-coated gold-silver nanocages and Yb-2, 4-dimethoxyhematoporphyrin: multifunctional capability of IR-luminescence detection, photosensitization, and photo-thermolysis. ACS Nano 5:7077–7089. doi:10.1021/nn2017974
Chen YC, Huang XC, Luo YL et al (2013) Non-metallic nanomaterials in cancer theranostics: a review of silica- and carbon-based drug delivery systems. Sci Technol Adv Mater 14:1–23. doi:10.1088/1468-6996/14/4/044407
Kim JW, Galanzha EI, Shashkov EV et al (2009) Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat Nanotechnol 4(10):688–694. doi:10.1038/nnano.2009.231
Ahamed M, Akhtar MJ, Siddiqui MA et al (2011) Oxidative stress mediated apoptosis induced by nickel ferrite in cultured A549 cells. Toxicology 283(2–3):101–108. doi:10.1016/j.tox.2011.02.010
Wu CH, Sokolov K (2014) Synthesis of immunotargeted magneto-plasmonic nanoclusters. J Vis Exp (90). doi: 10.3791/52090
Yu MK, Park J, Jon S (2012) Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2:3–34
Lin FH, Doong RA (2010) Synthesis of ferrite nanoparticle and ferrite-gold heterostructures. Adv Mater Res 123–125:251–255. doi:10.4028/www.scientific.net/AMR.123-125.251
Melancon MP, Elliott A, Ji X et al (2011) Theranostics with multifunctional magnetic gold nanoshells: photothermal therapy and t2* magnetic resonance imaging. Investig Radiol 46(2):132–140. doi:10.1097/RLI.0b013e3181f8e7d8
Balivada S, Rachakatla RS, Wang H et al (2010) A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: a mouse study. BMC Cancer 10:119–127. doi:10.1186/1471-2407-10-119
Glusac EJ (2012) The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol 39(1):17–20. doi:10.1111/j.1600-0560.2010.01660.x
Abdeen S, Praseetha PK (2013) Diagnostics and treatment of metastatic cancers with magnetic nanoparticles. J Nanomedine Biotherapeutic Discov 3:115. doi:10.4172/2155-983X.1000115
Edrei Y, Gross E, Corchia N et al (2011) Vascular profile characterization of liver tumors by magnetic resonance imaging using hemodynamic response imaging in mice. Neoplasia 13(3):244–253
Lencioni R, Cioni D, Crocetti L et al (2005) Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 234(3):961–967
Lau WY, Lai EC (2009) The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg 249(1):20–25. doi:10.1097/SLA.0b013e31818eec29
Liang P, Dong B, Yu X et al (2005) Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 235(1):299–307
Hinshaw JL, Lee FT Jr (2007) Cryoablation for liver cancer. Tech Vasc Interv Radiol 10(1):47–57. doi:10.1053/j.tvir.2007.08.005
Robinson JT, Welsher K, Tabakman SM et al (2010) High performance in vivo near-IR (>1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res 3(11):779–793. doi:10.1007/s12274-010
Acknowledgments
We would like to thank the Research Councils of Shiraz University of Medical Sciences (7803) for supporting this research and the Research Consultation Center, Shiraz University of Medical Sciences. The authors would also like to acknowledge Mr. Kouhi, Mr. Dideban-Mehr, and Mrs. Esfandiari for their efforts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
This study was conducted according to the Committee on the Ethics of Animal Experiments of Shiraz University of Medical Sciences.
Rights and permissions
About this article
Cite this article
Heidari, M., Sattarahmady, N., Azarpira, N. et al. Photothermal cancer therapy by gold-ferrite nanocomposite and near-infrared laser in animal model. Lasers Med Sci 31, 221–227 (2016). https://doi.org/10.1007/s10103-015-1847-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1847-x