Abstract
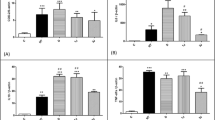
Low-level laser therapy is commonly used to treat tendinopathy or tendon injury. Tendon healing requires tenocyte migration to the repair site, followed by proliferation and synthesis of the extracellular matrix. There are few evidence to elucidate that low-level laser promote tenocyte proliferation. This study was designed to determine the effect of laser on tenocyte proliferation. Furthermore, the association of this effect with secretion of nitric oxide (NO) and the expressions of proliferating cell nuclear antigen (PCNA) and cyclins D1, E, A, and B1 was investigated. Tenocytes intrinsic to rat Achilles tendon were treated with low-level laser (660 nm). Tenocyte proliferation was evaluated by MTT assay and immunocytochemistry with Ki-67 stain. NO in the conditioned medium was measured by ELISA. Western blot analysis was used to evaluate the protein expressions of PCNA and cyclins D1, E, A, and B1. The results revealed that tenocytes proliferation was enhanced dose dependently by laser. NO secretion was increased after laser treatment. PCNA and cyclins E, A, and B1 were upregulated by laser. In conclusion, low-level laser irradiation stimulates tenocyte proliferation in a process that is mediated by upregulation of NO, PCNA, and cyclins E, A, and B1.





Similar content being viewed by others
References
Goldman JA (1981) Investigative studies of laser technology in rheumatology and immunology. In: Goldman L (ed) The biomedical laser: technology and clinical applications. Springer-Verlag, New York, p 293
Basford JR, Baxter GD (2010) Therapeutic physical agents. In: Frontera WR (ed) DeLisa’s physical medicine and rehabilitation, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1706–1707
Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD (2010) Low level laser treatment of tendinopathy: a systematic review with meta-analysis. Photomed Laser Surg 28:3–16
Bjordal JM, Lopes-Martins RA, Joensen J, Couppe C, Ljunggren AE, Stergioulas A, Johnson MI (2008) A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow). BMC Musculoskelet Disord 9:75
Stergioulas A, Stergioula M, Aarskog R, Lopes-Martins RA, Bjordal JM (2008) Effects of low-level laser therapy and eccentric exercises in the treatment of recreational athletes with chronic achilles tendinopathy. Am J Sports Med 36:881–887
Saunders L (2003) Laser versus ultrasound in the treatment of supraspinatus tendinosis: randomised controlled trial. Physiotherapy 89:365–373
Casalechi HL, Nicolau RA, Casalechi VL, Silveira L Jr, De Paula AM, Pacheco MT (2009) The effects of low-level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci 24:659–665
Oliveira FS, Pinfildi CE, Parizoto NA, Liebano RE, Bossini PS, Garcia EB, Ferreira LM (2009) Effect of low level laser therapy (830 nm) with different therapy regimes on the process of tissue repair in partial lesion calcaneous tendon. Lasers Surg Med 41:271–276
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, Gonzalez-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37:293–300
Nouruzian M, Alidoust M, Bayat M, Bayat M, Akbari M (2013) Effect of low-level laser therapy on healing of tenotomized Achilles tendon in streptozotocin-induced diabetic rats. Lasers Med Sci 28:399–405
Guerra Fda R, Vieira CP, Almeida MS, Oliveira LP, de Aro AA, Pimentel ER (2013) LLLT improves tendon healing through increase of MMP activity and collagen synthesis. Lasers Med Sci 28:1281–1288
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B 49:1–17
Lane N (2006) Cell biology: power games. Nature 443:901–903
Tafur J, Mills PJ (2008) Low-intensity light therapy: exploring the role of redox mechanisms. Photomed Laser Surg 26:323–328
Chen CH, Tsai JL, Wang YH, Lee CL, Chen JK, Huang MH (2009) Low-level laser irradiation promotes cell proliferation and mRNA expression of type I collagen and decorin in porcine Achilles tendon fibroblasts in vitro. J Orthop Res 27:646–650
Alexandratou E, Yova D, Handris P, Kletsas D, Loukas S (2002) Human fibroblast alterations induced by low power laser irradiation at the single cell level using confocal microscopy. Photochem Photobiol Sci 1:547–552
Lubart R, Breitbart H (2000) Biostimulative effects of low-energy lasers and their implications for medicine. Drug Dev Res 50:471–475
Sherr CJ (1996) Cancer cell cycles. Science 274:1672–1677
Pines J (1994) Protein kinases and cell cycle control. Semin Cell Biol 5:399–408
Prelich G, Stillman B (1988) Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell 53:117–126
Morris GF, Mathews MB (1989) Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem 264:13856–13864
Sachs F (1991) Mechanical transduction by membrane ion channels: a mini review. Mol Cell Biochem 104:57–60
Tsai WC, Hsu CC, Tang FT, Chou SW, Chen YJ, Pang JH (2005) Ultrasound stimulation of tendon cell proliferation and upregulation of proliferating cell nuclear antigen. J Orthop Res 23:970–976
O’Brien M (1992) Functional anatomy and physiology of tendons. Clin Sports Med 11:505–520
Leadbetter WB (1992) Cell-matrix response in tendon injury. Clin Sports Med 11:533–578
Murrell GA, Szabo C, Hannafin JA, Jang D, Dolan MM, Deng XH, Murrell DF, Warren RF (1997) Modulation of tendon healing by nitric oxide. Inflamm Res 46:19–27
Bokhari AR, Murrell GA (2012) The role of nitric oxide in tendon healing. J Shoulder Elbow Surg 21:238–244
Nichols SP, Storm WL, Koh A, Schoenfisch MH (2012) Local delivery of nitric oxide: targeted delivery of therapeutics to bone and connective tissues. Adv Drug Deliv Rev 64:1177–1188
Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR (2010) Role of low-level laser therapy in neurorehabilitation. Phys Med Rehabil 2:S292–S305
Girard F, Strausfeld U, Fernandez A, Lamb NJ (1991) Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell 67:1169–1179
Minshull J, Pines J, Golsteyn R, Standart N, Mackie S, Colman A, Blow J, Ruderman JV, Wu M, Hunt T (1989) The role of cyclin synthesis, modification and destruction in the control of cell division. J Cell Sci Suppl 12:77–97
Xiong Y, Zhang H, Beach D (1992) D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 71:505–514
Bjordal JM, Couppe C, Ljunggren AE (2001) Low level laser therapy for tendinopathy: evidence of a dose response. Phys Ther Rev 6:91–99
Acknowledgments
We would like to thank the National Science of Council, Taiwan, for the financial support of this research (grant no. 99-2314-B-182A-108).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, WC., Cheng, JW., Chen, JL. et al. Low-level laser irradiation stimulates tenocyte proliferation in association with increased NO synthesis and upregulation of PCNA and cyclins. Lasers Med Sci 29, 1377–1384 (2014). https://doi.org/10.1007/s10103-014-1528-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1528-1