Abstract
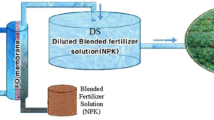
The objective of this research is to test the feasibility of a large-scale application of fertilizer drawn forward osmosis using a concentrated hydroponic nutrient solution as the draw solution. This large-scale application will help to mitigate the ever-growing issue of freshwater resources, by providing an alternative source of freshwater for agriculture. This method can then be used to grow crops hydroponically in arid regions that have access to both brackish groundwater and freshwater. The nutrient solution was prepared by dissolving industrial-grade fertilizer salts in dechlorinated tap water and was tested as a draw solution (DS) on a pilot-scale forward osmosis membrane module. The feed solution (FS) for the process was NaCl dissolved in dechlorinated tap water, in a 10 g/L (10% w/w) NaCl concentration to represent the salinity level of brackish groundwater. The performance of the draw solution was measured based on the water flux, water recovery, specific solute flux, and salt rejection. Two hydroponic basins were utilized to hydroponically grow lettuce. The first basin was grown using a commercial hydroponic nutrient solution, while the other basin was grown using the diluted draw solution from the pilot-scale. The growth of the lettuce in both basins was compared based on wet and dry weights of the stem, percent reduction in weight by drying, total length, stem length, and the ratio between stem and total lengths. Results showed that average water flux on the pilot-scale was found to be 1.11 L/m2/hr (LMH), while it was found to be 7.7 LMH in previous research on the bench-scale; this is in line with previous experiments conducted on both bench-scale and pilot-scale forward osmosis. The highest value specific reverse solute flux on the pilot-scale was found to be 7.48 g/m2/h for the K+ ion, which is also comparable to the results obtained on the bench-scale. Finally, salt rejection was found to be high, the lowest value was 90.7%, indicating high membrane selectivity against feed ions. The lettuce grown hydroponically using the resultant diluted draw solution from the pilot-scale was comparable to the lettuce grown using a commercial nutrient solution, with the draw solution lettuce being 16.23% less in terms of weight than the commercial lettuce, and 27.18% in stem length. This decrease in size and weight of the lettuce grown with the diluted draw solution can be attributed to the ratio of macro- and micro-nutrients in the Resh Florida/California solution not being optimized for the species of lettuce grown (loose leaf). It was concluded that concentrated hydroponic nutrient solution is a valid draw solution for large-scale fertilizer drawn forward osmosis, and this application mitigates some of the drawbacks of fertilizer drawn forward osmosis and increases the feasibility of large-scale application of forward osmosis as a desalination technology.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is one of the most significant resources for agricultural production and is crucial to food security; irrigated agriculture accounts for around 20% of all cultivated land and 40% of world food output (The World Bank 2020). Agriculture is the world's largest consumer of freshwater ("blue" water), with irrigated agriculture accounting for about 90% of all diverted freshwater (Traitler et al. 2018). By 2050, it is projected that the agricultural demand of the global water supply will increase by 19%, due to population growth (Globalagriculture 2020).
In order to mitigate the issue of water scarcity, countries have to adopt water demand mitigation measures, such as water conservation, and improved efficiencies. While this could help to close the gap between the supply and demand of water resources, it needs to be coupled with supply enhancement strategies, in order to complete combat water scarcity (Jones et al. 2019). Advances in technology are allowing for more solutions to the water shortage problem to be implemented, and seawater and brackish water desalination are the most viable solutions in order to address the water shortage problem, especially for potable water applications (Burn et al. 2015). Water desalination with the purpose of providing a fresh, usable supply of water has been gaining traction almost exponentially since the 1960s, at which point the globally commissioned desalination capacity was less than 2 million m3/d. By 2016, that capacity has increased to over 88 million m3/d (Kucera 2019). Particularly, in areas in the Middle East such as Saudi Arabia, United Arab Emirates, and Kuwait, seawater desalination is the most vital and dependable source of freshwater (Khawaji, Kutubkhanah and Wie 2007). The number of desalination technologies of both sea and brackish water is significant, and many of them have already achieved commercialization, while others are either approaching commercialization or are at an advanced stage of development, such as membrane desalination (Burn et al. 2015). Desalination technologies are categorized into two main categories: thermal desalination processes, and membrane desalination processes. Conventional thermal based desalination techniques are: Multiple-Effect Evaporation, also known as Multiple-Effect Distillation (MED), Multi-Stage Flash Evaporation (MSF), MED with thermally driven vapor compressor (MED-TVC), and Single-Stage Evaporation with mechanically driven vapor compressor (MVC) (Kucera 2019). Membrane based techniques are: Ultrafiltration (UF), Nanofiltration (NF), Electrodialysis (ED), Reverse Osmosis (RO), and Forward Osmosis (FO).
While RO is considered the most commercially utilized form of membrane desalination, it is still hindered by some issues and challenges, which are high energy requirement, high capital investment, and membrane fouling (Shon, Phuntsho, Zhang and Surampalli 2015). Hence, recent research and development efforts have been directed at further developing forward osmosis.
Unlike RO which utilizes hydraulic pressure to achieve separation through the semipermeable membrane, FO utilizes the natural phenomenon of osmotic pressure gradient between the draw solution and feed solution to achieve separation (Lambrechts and Sheldon 2019). This means that the overall energy consumption of FO processes is much less than that of RO (Shon et al. 2015). Moreover, FO does not require as much pumps and accessories, which further decreases its capital cost, relative to RO (Shon et al. 2015). However, FO is limited by membrane technology, as well as the selection of draw solution. Moreover, FO has a major limitation; the resultant diluted draw solution from the process still contains the draw solutes, and is often not suitable for direct use. Hence, FO is often coupled with another desalination technology, such as RO or NF, if the desired end product is pure water (Shon et al. 2015).
One of the challenges that face FO is the separation of draw solutes from the draw solution after FO, which is a demanding process that consumes extra energy and resources. However, this could be bypassed by using a draw solution that can be used directly without the need for solute separation. One of these possible solutions is utilizing fertilizers as the draw solutes, where the diluted draw solution could be used directly for fertigation, in a process known as fertilizer drawn forward osmosis (FDFO) (Phuntsho et al. 2011). This way, the resultant diluted draw solution could theoretically be used directly without the need for any extra processing. However, that is not usually the case, since FO is limited by the concentration gradient between the feed and draw solutions, and flux ceases when osmotic equilibrium is reached. This means that the draw solution needs to be further diluted in order to be suitable for fertigation. Hence, further research is needed to determine which draw solutes/fertilizer salts are the most suitable for FDFO, and would require the least dilution after desalination. Phuntsho et al. investigated low energy desalination using fertilizer drawn forward osmosis in order to find a method of utilizing desalinated water directly for fertigation. It was found that while all soluble fertilizers are capable of generating osmotic pressure, the performance of the fertilizer draw solution in terms of RSF and flux varied widely depending on the type of fertilizers used (Phuntsho et al. 2011). Hence, practical experimentation is necessary in order to assess the viability of the different fertilizers as potential draw solutions for fertilizer drawn forward osmosis, and there is no theoretical method of comparing the performance of different fertilizer salts; this is the reason this research and similar research are very crucial to the development of FDFO.
Coupling FDFO with hydroponics has great potential. Hydroponics is defined as “a method of growing plants in a water-based, nutrient-rich solution”, and instead of using soil, the roots are supported using an inert medium, such as rockwool, perlite, peat moss, clay pellets, or vermiculite (FAO 2021). Hydroponic agriculture presents a valuable method of agriculture to grow fresh vegetables in countries that have few arable lands, and those that are very small in area, but still have a large population (Resh 2012). The advantages of hydroponic agriculture over soil culture are more efficient delivery of nutrients, availability in regions that have nonarable land, efficient use of water and fertilizers, and higher planting density, which translates to higher yields per acre (Resh 2012). On the other hand, the limitations of hydroponics are high initial capital cost, and complex nutritional demands (Resh 2012). In hydroponics, macro and micro nutrients are supplied to the plant through a hydroponic nutrient solution. In FDFO, the draw solution could be the hydroponic nutrient solution, which after dilution through forward osmosis, could be used directly or after minor dilution for hydroponic agriculture. Since hydroponic nutrient solutions are often prepared in high concentrations, in order to facilitate transportation, and are diluted before use, FDFO presents an alternative source of water for dilution of the nutrient solutions, through recovering water from unusable feed water sources, such as wastewater (Chekli et al. 2017a), brackish groundwater, seawater, or brine. Chekli et al. studied the potential of coupling pilot-scale FDFO with hydroponic agriculture for sustainable water reuse. The feed solution in this study was wastewater and the draw solution was a commercial hydroponic nutrient solution. It was found that hydroponic lettuce grown with the nutrient solution diluted through FDFO showed similar trends with the standard hydroponic treatments in terms of biomass and growth patterns (Chekli et al. 2017a). However, no study has been performed yet on coupling pilot-scale FDFO with hydroponic agriculture as a method of desalination of brackish groundwater.
Another issue of FDFO is that the product diluted draw solution still possesses high concentrations of nutrients, which are still not suitable for direct fertigation. This leads to a need to compliment FDFO with another desalination technology or dilution in order to reach the required low nutrient concentrations. FDFO can also be coupled with other desalination technologies in order to overcome some of the inherent challenges of the technology. In Phuntsho et al. 2013 the potential of utilizing nanofiltration as a pre-treatment for brackish groundwater in order to reduce its TDS was investigated. By coupling FDFO with nanofiltration, significant enhancement of the water extraction capacity of FDFO and hence, achieving much more reduced nutrient concentrations in the final product water, allowing for direct fertigation (Phuntsho et al. 2013).
Theory
Due to the transfer of solvent driven by the force of the osmotic pressure difference (Δπ), when a semi-permeable membrane separates between a high osmotic pressure draw solution and a lower osmotic pressure feed solution, the draw solution is diluted while the feed solution is simultaneously concentrated (Moody and Kessler 1976; Lee et al. 1981; as cited in Shon et al. 2015). The chemical potential difference of the solvent between the draw and feed solutions causes this osmotic pressure differential (Shon et al. 2015). Osmotic pressure (π) is defined as the pressure required to keep pure water from passing through a semi-permeable barrier and into a solution (Mulder 1996; as cited in Shon et al. 2015).
The solution-diffusion model is the most often used model for mass transport over a FO membrane; in FO process, the water flux (Jv) and solute flux ( Js) across the membrane can be calculated as follows (Lee et al. 1981, as cited in Shon et al. 2015):
where A and B are the water and solute permeability coefficients, respectively, of the membrane; \(\sigma\) is the reflection coefficient, which is generally assumed to be unity; \(\Delta P\) is the applied hydraulic pressure; ∆C is the solute concentration difference across the membrane. Since \(\Delta P\) in FO process is equal to zero, water flux can be expressed as follows:
where \({\pi }_{\mathrm{draw}}\) and \({\pi }_{\mathrm{feed}}\) are the osmotic pressures of the draw and feed solutions, respectively.
It's worth noting that the FO water flux in Eq. (3) only applies under ideal conditions (Moody and Kessler 1976, as cited in Shon et al. 2015). The membrane is presumed to be a symmetric thin active layer that is only permeable to water and entirely impermeable to solutes under ideal conditions (Shon et al. 2015). It is also assumed that perfect mixing is achieved at both sides of the membrane, i.e., there is no concentration polarization (Shon et al. 2015).
However, the experimental FO water flux is frequently substantially lower than the projected product of membrane water permeability and the bulk solution osmotic pressure difference, as provided by Eq. (3) (Lee et al. 1981; Cath et al. 2006; McCutcheon et al., 2006; as cited in Shon et al. 2015). This is mainly due to a phenomenon known as concentration polarization (Moody and Kessler 1976; as cited in Shon et al. 2015). Concentration polarization is an issue inherent to all membrane liquid separation processes (Sablani et al. 2001; as cited in Shon et al. 2015). When a driving force is applied to a membrane, i.e., water is moved across the membrane under hydraulic pressure for pressure-driven membrane processes or osmotic pressure difference for FO, whereas feed solute movement is hampered; these held solutes collect at the membrane surface, forming a boundary layer (Mulder 1996; as cited in Shon et al. 2015). This phenomenon of “hampered component accumulating and permeating components being depleted at the membrane surface is referred to as concentration polarization” (Mulder 1996; Strathmann 1981; as cited in Shon et al. 2015).
When solutes exist in both feed and draw solutions, concentration polarization happens externally on both sides of the membrane in the FO process (Shon et al. 2015). Concentrative external concentration polarization (concentrative ECP) occurs when solutes accumulate on the feed side of the membrane, whereas dilutive external concentration polarization occurs when solutes on the draw side are flushed away by the flow of water, resulting in the depletion of draw solutes (dilutive ECP) (McCutcheon and Elimelech 2006; as cited in Shon et al. 2015). ECP causes an increase in osmotic pressure at the feed side and a drop in osmotic pressure at the draw side, resulting in a reduction in driving force (∆π) and a lower water flux than estimated by Eq. (3) (Shon et al. 2015).
However, when compared to ECP, internal concentration polarization (ICP) is a more important factor in the low FO water flux (Shon et al. 2015). Perfect mixing on the membrane surface is obtained in an ideal circumstance, homogenizing the solutions and mitigating the effects of concentration polarization (Shon et al. 2015). However, concentration polarization happens within the membrane under FO circumstances; semi-permeable membranes typically have two layers: a larger and porous support layer to provide appropriate handling strength and a thin selective layer (McCutcheon et al. 2005; as cited in Shon et al. 2015). Because this support layer is resistant to mass diffusion, a concentration polarization layer forms within it; this phenomenon is known as internal concentration polarization and is unique to osmotically driven membrane processes (Mehta and Loeb 1978; Loeb et al. 1997; McCutcheon and Elimelech 2006; as cited in Shon et al. 2015).
The active layer rejection layer of the asymmetric semi-permeable membrane can be arranged toward the draw solution (AL-DS) or the feed solution in FO operations (AL-FS). In an AL-DS orientation, water is transferred from the feed solution through the membrane's rejection layer, while feed solutes are transferred into the support layer via convection; the rejection layer's retention of the feed solutes causes a boundary layer to form in the support section of the membrane (Shon et al. 2015). In the meantime, the support layer prevents the solutes from diffusing back to the bulk phase, a phenomenon known as concentrative ICP (McCutcheon et al. 2005; McCutcheon and Elimelech 2006; as cited in Shon et al. 2015). In an AL-FS orientation, the draw solution in the support layer is diluted as water passes through the rejection layer (Shon et al. 2015). To reestablish the osmotic driving forces, draw solutes must diffuse toward the rejection-layer-support interface. However, the support layer obstructs diffusion, resulting in a depletion of draw solutes along the rejection-layer-support interface, causing a phenomenon known as dilutive ICP (McCutcheon et al. 2005; McCutcheon and Elimelech 2006; as cited in Shon et al. 2015 Water flux is reduced in both concentrative and dilutive ICP events. The ICP phenomenon can be summarized as the membrane support layer obstructing solute diffusion (Lee et al. 1981; Loeb et al. 1997; Loeb 2002; Gray et al. 2006; Tang et al. 2010; as cited in Shon et al. 2015).
One of the most significant characteristics to consider in FO processes is reverse solute flux (RSF). Due to the loss of draw solutes into the feed solution that cannot be recovered, reverse diffusion of draw solutes into the feed solution represents an economic loss (Cath et al. 2006; as cited in Nasr and Sewilam 2016). Due to the formation of complexes between feed and draw ions, reverse solute flux increases the occurrence of membrane fouling, in addition to the loss of draw solutes (Cath et al. 2006; Lay et al. 2010; as cited in Nasr and Sewilam 2016). The FO reverse solute flux can be determined by (Tang et al. 2010; as cited in Shon et al. 2015):
where \(\beta\) is the van’t Hoff coefficient; \({R}_{g}\) is the universal gas constant; T is the absolute temperature.
Specific reverse solute flux (SRSF) is defined as the ratio between reverse solute flux and water flux, and it is a unit of measurement for the mass of draw solutes lost due to reverse permeation per unit volume of water claimed from the FS. (Cath et al. 2006; as cited in Nasr and Sewilam 2015). SRSF can be calculated as follows:
By combining Eqs. (4) and (5), SRSF can be expressed as:
Materials and methods
Pilot-scale FDFO
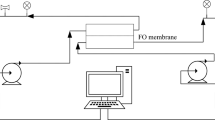
For this experiment, a pilot-scale crossflow filtration unit with an FO membrane module was used, illustrated in Fig. 1. The membrane module (PFO-100) was obtained from Porifera, and has a membrane area of 7 m2 (Porifera Inc. 2015). The PFO-100 is a flat sheet, 8-port system consisting of two independent fluid channels separated by Porifera’s proprietary membrane (Porifera Inc. 2015). The membrane module specifications are elaborated in Table 1. The module has two sides, one dedicated for input connections and the other for output connections. Each side has four ports; two for FS and two for DS, as demonstrated in Fig. 2.
Ports of a Porifera FO membrane module (Porifera Inc. 2015)
Two ports on each side of the membrane were connected to pressure gages, in order to monitor the pressures of both the input and output ports of the FS and DS, and ensure that they do not exceed the maximum pressure limit of the membrane. The crossflow was achieved by utilizing two 0.55 kW circulation pumps, and each pump was connected to feed and draw tanks, in order to ensure continuous flow of solutions from the tanks to the membrane and back to the tanks. The draw solution tank was placed on a platform scale, in order to record the change in the weight of the tank. The scale was connected to a data logging software, in order to record the change in weight.
Water flux, reverse solute flux, and salt rejection
Water flux Jv (LMH) was calculated using
Reverse solute flux (g/m.2/h) was calculated using
where \({V}_{i}\) is the initial volume of FS, \(\Delta V\) is the total volume of water displaced from the FS to the DS, \({C}_{s}\) is the concentration of the draw solutes in the FS at the end of the experiment, Sa is the membrane surface area, and t is time.
Moreover, salt rejection is also an important property of FO membranes, and was investigated by analyzing the Na+ and Cl.− ions in the resultant DS from each experiment, and calculated using the following equation
where \({C}_{i}\) is the initial concentration of the ion in FS, \({C}_{p,D}\) is the final concentration of the ion in DS, \({V}_{i}\) is the initial volume of the DS and \(\Delta V\) is the total volume of water that entered the DS from the FS (Nasr and Sewilam 2016).
Hydroponic nutrient solution
The nutrient solution utilized in this experiment as a draw solution for FO is the “Resh Florida/California Lettuce” (RF) solution (Resh 2012). This solution was chosen based on previous experiments done on the bench-scale, in order to compare between the performance of six different hydroponic nutrient solutions as draw solutions for fertilizer drawn forward osmosis (Bassiouny et al. 2022). The RF solution was found to be among the highest performing solution among those tested, in terms of water flux, reverse solute flux, and salt rejection, as well as having a relatively low price per liter, when compared to the other solutions. Shown in Table 2 are the macro- and micro-nutrients composition of the Resh Florida/California solution (Resh 2012).
The 2 parts of the nutrient solution were prepared by dissolving salts in dechlorinated tap water, in 100 strength stock solutions. Shown in Table 3 are the salts that were used. Water was dechlorinated by evaporation, where the water was left exposed to the air prior to the experiments for at least 24 h, in order to guarantee full evaporation of the chlorine. While dechlorination can be carried out by using chemicals such as Sodium Thiosulfate (Na2S2O3) or Sodium Metabisulfite (Na2S2O5), evaporation was utilized in order to avoid using chemicals in the investigation, which might affect the results (El Zayat et al. 2021).
Hydroponic lettuce
Loose leaf (Batavia) lettuce seeds were obtained from Urban Greens, Egypt. Moreover, a commercial hydroponic nutrient solution was procured, in order to compare between the performance of the diluted draw solution and the commercial nutrient solution. The commercial nutrient solution utilized was also obtained from Urban Greens, Egypt. Two nutrient film technique (NFT) hydroponic systems were utilized in this experiment, each with a capacity of 120 seedlings, and a nutrient tank capacity of 60L. Seeds were germinated in a separate nursery for 30 days prior to being moved to the hydroponic systems.
Experimental plan
Pilot-scale fertilizer drawn forward osmosis
Feed solution was prepared by dissolving industrial-grade NaCl in dechlorinated tap water in 10 g/L (10% w/w) concentration. This concentration represents the average salinity of brackish groundwater. Draw solution was prepared by dissolving industrial-grade salts in dechlorniated tap water. All salts were completely dissolved in water utilizing an overhead stirrer.
Three replications of the experiment were performed, in order to verify the obtained results, where the feed solution was 10 g/L NaCl solution and the draw solution was 100 strength part B solution of the Resh/Florida lettuce hydroponic nutrient solution. Part B was chosen as the draw solution for the process due to the existence of high concentrations of Ca in part A, which could lead to membrane fouling (Chekli et al. 2017a). Moreover, two replications of a baseline experiment were performed. In the baseline experiment, the feed solution was dechlorinated tap water and the draw solution was the hydroponic nutrient solution. Baseline experiments were performed in order to investigate the maximum obtainable water flux from the system, and compare it with the other experiments.
For each experiment, 200L of FS and 20L of DS were placed in their respective tanks. Initial TDS was measured by using a portable TDS and EC meter (Hach HQ40D multi). The pilot-scale system was then run for 3.5 to 4 h. Change in the weight of the DS tank was recorded through the platform scale and data logging software at 2 min intervals. Afterward, samples were taken from both solutions, and the draw solution was drained into another tank, for use in hydroponic agriculture of lettuce. Then, the system was flushed for 3 times with dechlorinated tap water, which was then drained from the system. During the duration of the experiment, any external sources that could cause heat variation (air conditioners) were turned off, in order to minimize any variation in the temperature of the solutions, which would potentially have an effect on the flux.
The ions in each of the FS and DS samples from each experiment were analyzed using spectrophotometry (Spectroquant Nova 60 A), in order to determine their ionic composition.
Hydroponic agriculture of lettuce
Two Nutrient Film Technique (NFT) hydroponic systems were utilized, each with a capacity of 120 plants. The resulting draw solution from the pilot-scale FDFO was mixed with part A of the solution, and then diluted in the nutrient tank of the hydroponic basin to a similar electrical conductivity (EC) as that of the commercial nutrient solution; this was done to eliminate EC as a parameter in the growth of the lettuce. It was found that the appropriate dilution ratio for the resultant DS was 75:1, while the dilution ratio for the commercial nutrient solution was 200:1, as per the manufacturer’s instructions. The EC for both hydroponic basins was 2.75. Then, the Resh Florida/California solution was used to grow the lettuce in one of the basins. The other basin was grown using the commercial nutrient solution, as a control.
The lettuce was harvested from the hydroponic basins after 6 weeks, and measurements were obtained from 10 samples from each basin. The measurements were taken for the stem wet weight, total length, and stem length. Then, the samples were dried in an oven for 72 h at a temperature of 60 °C, and the dry weight of the stems was recorded. Finally, the ratios between the stem wet weight and dry weight, and between the stem length and total length were calculated, as well as the averages for all the measurements.
Results and discussion
Pilot-scale FDFO
Water flux
The average volume of water recovered from the FS by the DS was 20.7 L, which represents a dilution of 103.5%. The TDS of both draw and feed solutions was measured before and after each experiment, and the average value is demonstrated in Fig. 3. TDS in FS increased by 11.73% on average, while TDS in DS decreased by 70.26% on average. Equation (7) was used to calculate the water flux for all experiments, and the results are demonstrated in Figs. 4 and 5. Figure 4 demonstrates the flux behavior over the duration of the experiment for the baseline experiments, while Fig. (5) demonstrates the behavior for the three replications of the 10 g/L NaCl FS vs Resh Florida/California nutrient solution DS. The average water flux was taken as the value of flux at the point of flux stabilization. The average flux in the baseline experiments was found to be around 3.39 LMH in both baseline experiments. As expected, flux stabilized after 40 min, which is consistent with previous experiments in FDFO. The average water flux for the three replications was found to be 1.11 LMH. This is considerably lower than the results obtained on the bench-scale, where the average flux for the same solutions was 7.7 LMH (Bassiouny et al. 2022). This is consistent with the results of previous experiments on both the bench and pilot-scales, such as in El Zayat, where the flux on the bench-scale was found to be 21.48 LMH, while on the pilot-scale the flux was 1.52 LMH, for the same FS and DS (synthetic brine and ammonium sulfate solution, respectively)(El Zayat et al. 2021). This decrease in flux between bench-scale and pilot-scale apparatuses could be attributed to the difference in flow rate between the pump systems of both apparatuses, relative to the membrane area; the flow rate in the previous bench scale experiments was 0.4 L/min for a membrane area of 0.00127 m2 while in pilot scale the flow rate was 11.8 L/min for a membrane area of 7 m2. The decrease in flux between the bench-scale and pilot-scale can also be due to the occurrence of higher ICP and ECP in the larger membrane surface area.
Specific Reverse Solute Flux
Equation 8 was used to calculate the reverse solute flux for all the ions in the DS, and the average reverse solute flux was demonstrated in Fig. 6. Specific reverse solute flux (SRSF) was calculated from Eqs. (5), (7), and (8) for all the ions in the DS (SO42−, Mg2+, K+, PO43−, NH4+), and the average SRSF was demonstrated in Fig. 7. It was observed that the highest SRSF were observed in K+, NH4+, and SO42−, with the highest values recorded being 7.48 g/m2/h, 2.82 g/m2/h, and 3.2 g/m2/h, respectively. It should be noted that low SRSF values indicate high membrane selectivity. The values of SRSF obtained are within the range of values obtained in previous experiments on the bench-scale (Bassiouny et al. 2022). It should be noted that any discrepancies in the values of SRSF between bench-scale and pilot-scale experiments can be attributed to the slight modification of chemicals used between both in the preparation of the DS. This slight modification was necessary due to restrictions in availability of some industrial-grade chemicals. Reverse solute permeation could be attributed to both dilutive ECP and ICP phenomena, due to the orientation of the membrane (AL-FS), which could have caused a build-up of draw ions on the draw side of the membrane, generating a driving force across the membrane. The membrane performance was measured after the conclusion of all the experiments, and no noticeable decline in the performance of the membrane was observed, indicating that no significant biofouling occurred throughout the duration of the experiments.
Salt rejection
Permeation of FS ions (Na+ and Cl−) into the DS was calculated using Eq. (9), and the results are demonstrated in Fig. 8. It can be observed from the figure that a high rejection of FS ions was achieved in all replications of the experiment, with the lowest value being 90.7%, and the highest being 93.81%. This indicates that the membrane utilized in the experiments was highly selective against FS ions, which is the desirable condition in order to avoid contamination of DS with FS ions. This is especially crucial in FDFO, since FS ions (Na+ and Cl−) can affect the growth of plants grown using the diluted DS.
Hydroponic agriculture of lettuce
Results for the measurements of the lettuce grown hydroponically with both commercial nutrient solution and the diluted Resh Florida/California DS are demonstrated in Fig. 9.
Table 4 represents the ratio between the averages in measurements of the commercial nutrient solution and the Resh/Florida solution in terms of percentages. The highest ratio was between the stem dry weights, while the lowest was observed in total length. It can be observed that the measurements for the lettuce grown using the diluted DS (Resh Florida/California) were lower, which indicates that the net yield was lower in this hydroponic basin. This can be explained by the ratio of macro- and micro-nutrients in the DS, which might have not been optimal for this specific species of lettuce, and the growing conditions in which the lettuce was grown. This could also be explained by the presence of minor amounts of Na+ and Cl− ions in the diluted draw solution, which might have affected the growth of the lettuce. The ratio of macro- and micro-nutrients in the commercial nutrient solution has been investigated, but was not included in this paper based on the request of the solution supplier.
Conclusion
This study investigated the performance of the Resh Florida/California hydroponic nutrient solution as a draw solution for fertilizer drawn forward osmosis on the pilot-scale. The performance was assessed based on water flux, specific reverse solute flux, and salt rejection, and was compared to results obtained from the bench-scale. The feed solution for the process was 10 g/L NaCl in tap water, as a form of synthetic brackish groundwater. The resultant diluted draw solution from pilot-scale forward osmosis was then utilized to hydroponically grow lettuce, and the resultant lettuce was compared against a control group grown using a commercial hydroponic nutrient solution. The study was carried out in order to further assess the viability of utilizing fertilizer drawn forward osmosis on a bigger scale, in order to be applied in arid regions, and provide an alternative source of water for agriculture through desalination of brackish groundwater.
The highest average water flux was found to be 1.11 LMH, and the lowest recorded average was 0.94 LMH among the three replications of the experiment; the baseline flux was 3.39 LMH. SRSF values for DS ions were calculated and the highest value was 6.73 g/m2/h, and was recorded in the K+ ion. Finally, salt rejection was calculated, and the lowest percent rejection was 90.7%, and was recorded for Cl− ion in the first replication of the experiment. The diluted DS resulting from the pilot-scale experiments was used to hydroponically grow lettuce, and the properties of the grown lettuce were compared against a control group grown using a commercial hydroponic nutrient solution. It was found that the lettuce grown using the commercial nutrient solution was overall larger in size (27.18% on average) and weight (16.23% on average) than the one grown with the diluted Resh Florida/California solution, which can be attributed to the ratio of macro and micronutrients in the Resh solution not being optimized for the species of lettuce grown. However, the yield was still comparable, which demonstrates that utilizing forward osmosis to dilute hydroponic nutrient solutions is a valid method of providing an alternative source of water for agriculture.
It can be concluded that concentrated hydroponic nutrient solution is a valid draw solution for large-scale fertilizer drawn forward osmosis, and this application mitigates some of the drawbacks of fertilizer drawn forward osmosis and increases the feasibility of forward osmosis as a desalination technology. Moreover, the hydroponic nutrient solution did not have any negative effect on the performance of the membrane when used as a draw solution. It can also be concluded that reverse solute flux had minimal negative effect on the growth of crops grown hydroponically using the diluted draw solution from FDFO, and that this model can be applied on a larger scale in an arid region that has access to both freshwater and brackish groundwater.
Future research efforts should be directed at testing the performance of real brackish groundwater samples as FS, experimenting with different hydroponic nutrient solutions draw solutions and investigating their performance on the pilot-scale, growing crops other than lettuce and comparing them with crops grown with dilution through forward osmosis, utilizing the most recently developed forward osmosis membranes, and combining forward osmosis with another different technique of desalination (such as reverse osmosis or nanofiltration) as a source of the remaining water required for dilution of DS, and performing a feasibility study on such combination of technologies.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bassiouny M, Nasr P, Sewilam H (2022) Investigating the performance of hydroponic nutrient solutions as potential draw solutions for fertilizer drawn forward osmosis. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-18701-5
Burn S, Hoang M, Zarzo D, Olewniak F, Campos E, Bolto B, Barron O (2015) Desalination techniques — a review of the opportunities for desalination in agriculture. Desalination 364:2–16. https://doi.org/10.1016/j.desal.2015.01.041
Cath TY, Childress AE, Elimelech M (2006) Forward osmosis: principles, applications, and recent developments. J Membr Sci 281(1–2):70–87. https://doi.org/10.1016/j.memsci.2006.05.048
Chekli L, Kim JE, El Saliby I, Kim Y, Phuntsho S, Li S, Shon HK (2017a) Fertilizer drawn forward osmosis process for sustainable water reuse to grow hydroponic lettuce using commercial nutrient solution. Sep Purif Technol 181:18–28. https://doi.org/10.1016/j.seppur.2017.03.008
Chekli L, Kim Y, Phuntsho S, Li S, Ghaffour N, Leiknes TO, Shon HK (2017b) Evaluation of fertilizer-drawn forward osmosis for sustainable agriculture and water reuse in arid regions. J Environ Manage 187:137–145. https://doi.org/10.1016/j.jenvman.2016.11.021
El Zayat H, Nasr P, Sewilam H (2021) Investigating sustainable management of desalination brine through concentration using forward osmosis. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-13311-z
FAO (2021) Home gardens/vertical farming, hydroponics and aquaponics. Retrieved June 30, 2022, from https://www.fao.org/land-water/overview/covid19/homegardens/fr/
Globalagriculture (2020) Water. Retrieved November 18, 2020, from https://www.globalagriculture.org/report-topics/water.html
Gray GT, McCutcheon JR, Elimelech M (2006) Internal concentration polarization in forward osmosis: role of membrane orientation. Desalination 197(1–3):1–8
Jones E, Qadir M, Van Vilet MT, Smakhtin V, Kang S (2019) The state of desalination and brine production: a global outlook. Sci Total Environ 657:1343–1356. https://doi.org/10.1016/j.scitotenv.2018.12.076
Khawaji AD, Kutubkhanah IK, Wie J (2008) Advances in seawater desalination technologies. Desalination 221(1–3):47–69. https://doi.org/10.1016/j.desal.2007.01.067
Kucera, J. (2019). Desalination: Water from water. ProQuest Ebook Central https://ebookcentral.proquest.com
Lambrechts R, Sheldon MS (2019) Performance and energy consumption evaluation of a fertiliser drawn forward osmosis (FDFO) system for water recovery from brackish water. Desalination 456:64–73. https://doi.org/10.1016/j.desal.2019.01.016
Lay WCL, Chong TH, Tang CY, Fane AG, Zhang J, Liu Y (2010) Fouling propensity of forward osmosis: investigation of the slower flux decline phenomenon. Water Sci Technol 61(4):927–936. https://doi.org/10.2166/wst.2010.835
Lee KL, Baker RW, Lonsdale HK (1981) Membranes for power generation by pressure-retarded osmosis. J Membr Sci 8(2):141–171
Loeb S (2002) Large-scale power production by pressure-retarded osmosis, using river water and sea water passing through spiral modules. Desalination 143(2):115–122
Loeb S, Titelman L, Korngold E, Freiman J (1997) Effect of porous support fabric on osmosis through a loeb-sourirajan type asymmetric membrane. J Membr Sci 129(2):243–249
McCutcheon JR, Elimelech M (2006) Influence of concentrative and dilutive internal concentration polarization on flux behaviour in forward osmosis. J Membr Sci 284(1–2):232–242
McCutcheon JR, McGinnis RL, Elimelech M (2005) A novel ammonia – carbon dioxide forward (direct) osmosis desalination process. Desalination 174(1):1–11
Mehta GD, Loeb S (1978) Internal polarization in the porous substructure of a semipermeable membrane under pressure-retarded osmosis. J Membr Sci 4:261–265
Moody, C. D. and Kessler, J. O. (1976). “Forward osmosis extractors.” Desalination, 18(3), 283 – 295. Mulder, M. (1996). Basic principles of membrane technology, Kluwer Academic Publishers. M. (2006). “Internal concentration membrane orientation.” Desalination
Mulder M (1996) Basic principles of membrane technology. Kluwer Academic Publishers, Dordrecht
Nasr P, Sewilam H (2015) Forward osmosis: an alternative sustainable technology and potential applications in water industry. Clean Technol Environ Policy 17(7):2079–2090. https://doi.org/10.1007/s10098-015-0927-8
Nasr P, Sewilam H (2016) Investigating the performance of ammonium sulphate draw solution in fertilizer drawn forward osmosis process. Clean Technol Environ Policy 18(3):717–727. https://doi.org/10.1007/s10098-015-1042-6
Phuntsho S, Shon HK, Hong S, Lee S, Vigneswaran S (2011) A novel low energy fertilizer driven forward osmosis desalination for direct fertigation: evaluating the performance of fertilizer draw solutions. J Membr Sci 375:172–181. https://doi.org/10.1016/j.memsci.2011.03.038
Phuntsho S, Hong S, Elimelech M, Shon HK (2013) Forward osmosis desalination of brackish groundwater: meeting water quality requirements for fertigation by integrating nanofiltration. J Membr Sci 436:1–15. https://doi.org/10.1016/j.memsci.2013.02.022
Porifera Inc (2015) PFO-100 User Manual (Version 2.3)
Resh, H. M. (2012). Hydroponic food production: A definitive guidebook for the advanced home gardener and the commercial hydroponic grower, seventh edition. ProQuest Ebook Central https://ebookcentral.proquest.com
Sablani S, Goosen M, Al-Belushi R, Wilf M (2001) Concentration polarization in ultra-filtration and reverse osmosis: a critical review. Desalination 141(3):269–289
Shon, H. K., Phuntsho, S., Zhang, T. C., & Surampalli, R. Y. (Eds.). (2015). Forward Osmosis: Fundamentals and Applications. American Society of Civil Engineers. Retrieved November 17, 2020, from https://ebookcentral.proquest.com
Strathmann H (1981) Membrane separation processes. J Membr Sci 9(1–2):121–189
Tang CY, She Q, Lay WCL, Wang R, Fane AG (2010) Coupled effects of internal concentration polarization and fouling on flux behavior of forward osmosis membranes during humic acid filtration. J Membr Sci 354(1–2):123–133
The World Bank. (2020, May 8). Water in Agriculture. https://www.worldbank.org/en/topic/water-in-agriculture.
Traitler H, Dubois MJ, Heikes K, Petiard V, and Zilberman D (2018). Megatrends in food and agriculture : Technology, water use and nutrition. ProQuest Ebook Central https://ebookcentral.proquest.com
Urban Greens Egypt (2020). https://www.urbangreensegypt.com
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding for this research was provided by the Center for Applied Research on the Environment and Sustainability, and the American University in Cairo.
Author information
Authors and Affiliations
Contributions
MB, PN and HS contributed to the conception of the study and its design. Material preparation, data collection and analysis were performed by MB. The manuscript draft was written by MB and reviewed by PN and by HS.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bassiouny, M., Nasr, P. & Sewilam, H. Investigating the pilot-scale performance of a hydroponic nutrient solution as potential draw solution for fertilizer drawn forward osmosis and hydroponic agriculture of lettuce. Clean Techn Environ Policy 24, 2749–2760 (2022). https://doi.org/10.1007/s10098-022-02349-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-022-02349-3