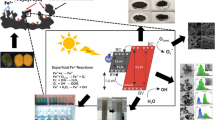
Abstract
Nanostructured magnesium oxide (MgO) catalysts were prepared by the coprecipitation method and employed for the transesterification of waste cooking oil using methanol. The X-ray diffraction analysis showed that nanostructured MgO phase was formed at calcination temperature of 500 °C. The mean crystallite size of MgO nanoparticles is 7.86 nm. Fourier-transformed infrared spectroscopy studies confirmed the formation of MgO phase with the characteristic vibrational mode of Mg–O. UV–Vis diffuse reflectance spectroscopy reveals that the energy band gap is around 5.84 eV. The presence of magnesium and oxygen elements was determined from energy-dispersive X-ray analysis. The effect of various parameters such as catalyst loading, methanol-to-oil molar ratio, reaction temperature, reaction time and reusability was investigated. A maximum biodiesel yield of 93.3% was achieved with 2 wt% of MgO nanocatalyst (MO5 sample), methanol/oil molar ratio of 24:1, reaction temperature about 65 °C and reaction time 1 h. The nanocatalyst (MgO) was reused at least for 5 times and thereafter resulted in a decrease in the biodiesel yield. The kinetic study of the transesterification reaction followed pseudo-first-order rate kinetics. The composition of fatty acid methyl ester was determined using gas chromatography–mass spectroscopy.













Similar content being viewed by others
Abbreviations
- WCO:
-
Waste cooking oil
- MH1:
-
Magnesium hydroxide at 100 °C
- MH2:
-
Magnesium hydroxide at 200 °C
- MH3:
-
Magnesium hydroxide at 300 °C
- MH4:
-
Magnesium hydroxide at 400 °C
- MO5:
-
Magnesium oxide at 500 °C
- FAME:
-
Fatty acid methyl ester
- XRD:
-
X-ray diffraction
- HR-SEM:
-
High-resolution scanning electron microscopy
- EDX:
-
Energy-dispersive X-ray diffraction
- FT-IR:
-
Fourier infrared spectroscopy
- DRS:
-
Diffuse reflecting spectroscopy
- SDS:
-
Sodium dodecyl sulphate
- X :
-
Conversion of triglyceride
- t :
-
Time in minutes
- wt:
-
Weight in grams
- %:
-
Percentage
- eV:
-
Electron volt
- R :
-
Diffuse reflectance
- σ :
-
Strain
- a, c :
-
Lattice constants
- D :
-
Average crystal size
- θ :
-
Angle in degree
- λ :
-
Wavelength
- °:
-
Degree
- hkl :
-
Miller indices
- TG:
-
Triglyceride
References
Amani H, Ahmad Z, Asif M, Hameed BH (2014) Transesterification of waste cooking palm oil by MnZr with supported alumina as a potential heterogeneous catalyst. J Ind Eng Chem 20:4437–4442. https://doi.org/10.1016/j.jiec.2014.02.012
Anand GT, Kennedy LJ, Vijaya JJ et al (2014) Structural, optical and magnetic characterization of Zn1−xNixAl2O4 (0 ≤ x ≤ 5) spinel nanostructures synthesized by microwave combustion technique. Ceram Int 41:603–615. https://doi.org/10.1016/j.ceramint.2014.08.109
Balakrishnan K, Olutoye MA, Hameed BH (2013) Synthesis of methyl esters from waste cooking oil using construction waste material as the solid base catalyst. Bioresour Technol 128:788–791. https://doi.org/10.1016/j.biortech.2012.10.023
Boro J, Thakur AJ, Deka D (2011) Solid oxide derived from waste shells of Turbonilla striatula as a renewable catalyst for biodiesel production. Fuel Process Technol 92:2061–2067. https://doi.org/10.1016/j.fuproc.2011.06.008
Chen GY, Shan R, Yan BB et al (2016) Remarkably enhancing the biodiesel yield from palm oil upon abalone shell-derived CaO catalysts treated by ethanol. Fuel Process Technol 143:110–117. https://doi.org/10.1016/j.fuproc.2015.11.017
Deshmane VG, Adewuyi YG (2013) Synthesis and kinetics of biodiesel formation via calcium methoxide base catalyzed transesterification reaction in the absence and presence of ultrasound. Fuel 107:474–482. https://doi.org/10.1016/j.fuel.2012.12.080
Devaraja PB, Avadhani DN, Prashantha SC et al (2014) Synthesis, structural and luminescence studies of magnesium oxide nanopowder. Spectrochim Acta Part A Mol Biomol Spectrosc 118:847–851. https://doi.org/10.1016/j.saa.2013.08.050
Dodabalapur A, Rothberg LJ, Fung WP, et al (1996) Nanorod-superconductor composites: a pathway to materials with high critical current densities. Science 273:1836–1840. https://doi.org/10.1126/science.273.5283.1836
Esmaeili E, Khodadadi A, Mortazavi Y (2009) Microwave-induced combustion process variables for MgO nanoparticle synthesis using polyethylene glycol and sorbitol. J Eur Ceram Soc 29:1061–1068. https://doi.org/10.1016/j.jeurceramsoc.2008.07.051
Farooq M, Ramli A, Subbarao D (2013) Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J Clean Prod 59:131–140. https://doi.org/10.1016/j.jclepro.2013.06.015
Feyzi M, Norouzi L (2016) Preparation and kinetic study of magnetic Ca/Fe3O4@SiO2nanocatalysts for biodiesel production. Renew Energy 94:579–586. https://doi.org/10.1016/j.renene.2016.03.086
Hadia NMA, Mohamed HAH (2015) Characteristics and optical properties of MgO nanowires synthesized by solvothermal method. Mater Sci Semicond Process 29:238–244. https://doi.org/10.1016/j.mssp.2014.03.049
Klabunde KJ, Stark J, Koper O et al (1996) Nanocrystals as stoichiometric reagents with unique surface chemistry. J Phys Chem 100:12142–12153. https://doi.org/10.1021/jp960224x
Li Y, Bando Y, Sato T (2002) Preparation of network-like MgO nanobelts on Si substrate. Chem Phys Lett 359:141–145. https://doi.org/10.1016/S0009-2614(02)00672-3
Liu X, He H, Wang Y, Zhu S (2007) Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal Commun 8:1107–1111. https://doi.org/10.1016/j.catcom.2006.10.026
Liu Y, Lu H, Ampong-Nyarko K et al (2016) Kinetic studies on biodiesel production using a trace acid catalyst. Catal Today 264:55–62. https://doi.org/10.1016/j.cattod.2015.07.004
Mageshwari K, Mali SS, Sathyamoorthy R, Patil PS (2013) Template-free synthesis of MgO nanoparticles for effective photocatalytic applications. Powder Technol 249:456–462. https://doi.org/10.1016/j.powtec.2013.09.016
Maroušek J, Itoh S, Higa O et al (2013) Pressure shockwaves to enhance oil extraction from Jatropha curcas L. Biotechnol Biotechnol Equip 27:3654–3658. https://doi.org/10.5504/BBEQ.2012.0143
Mazaheri H, Ong HC, Masjuki HH et al (2018) Rice bran oil based biodiesel production using calcium oxide catalyst derived from Chicoreus brunneus shell. Energy 144:10–19. https://doi.org/10.1016/j.energy.2017.11.073
Mirzaei H, Davoodnia A (2012) Microwave assisted sol–gel synthesis of MgO nanoparticles and their catalytic activity in the synthesis of Hantzsch 1,4-dihydropyridines. Chin J Catal 33:1502–1507. https://doi.org/10.1016/S1872-2067(11)60431-2
Moorthy SK, Ashok CH, Rao KV, Viswanathan C (2015) Synthesis and characterization of Mgo nanoparticles by neem leaves through green method. Mater Today Proc 2:4360–4368. https://doi.org/10.1016/j.matpr.2015.10.027
Puchert MK, Timbrell PY, Lamb RN (1996) Postdeposition annealing of radio frequency magnetron sputtered ZnO films. J Vac Sci Technol A Vac Surf Film 14:2220–2230. https://doi.org/10.1116/1.580050
Refaat AA (2010) Biodiesel production using solid metal oxide catalysts. Int J Environ Sci Technol 8:203–221. https://doi.org/10.1007/BF03326210
Rezaei M, Khajenoori M, Nematollahi B (2011) Preparation of nanocrystalline MgO by surfactant assisted precipitation method. Mater Res Bull 46:1632–1637. https://doi.org/10.1016/j.materresbull.2011.06.007
Román-Figueroa C, Olivares-Carrillo P, Paneque M et al (2016) High-yield production of biodiesel by non-catalytic supercritical methanol transesterification of crude castor oil (Ricinus communis). Energy 107:165–171. https://doi.org/10.1016/j.energy.2016.03.136
Saleh R, Djaja NF (2014) UV light photocatalytic degradation of organic dyes with Fe-doped ZnO nanoparticles. Superlattices Microstruct 74:217–233. https://doi.org/10.1016/j.spmi.2014.06.013
Selvam NCS, Kumar RT, Kennedy LJ, Vijaya JJ (2011) Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J Alloys Compd 509:9809–9815. https://doi.org/10.1016/j.jallcom.2011.08.032
Silitonga AS, Masjuki HH, Mahlia TMI et al (2013) Experimental study on performance and exhaust emissions of a diesel engine fuelled with Ceiba pentandra biodiesel blends. Energy Convers Manag 76:828–836. https://doi.org/10.1016/j.enconman.2013.08.032
Sivakumar P, Sankaranarayanan S, Renganathan S (2013) Studies on sono-chemical biodiesel production using smoke deposited nano MgO catalyst. Bull Chem React Eng Catal 8:89–96. https://doi.org/10.9767/bcrec.8.2.4628.89-96
Song G, Ma S, Tang G, Wang X (2010) Ultrasonic-assisted synthesis of hydrophobic magnesium hydroxide nanoparticles. Colloids Surf A Physicochem Eng Asp 364:99–104. https://doi.org/10.1016/j.colsurfa.2010.04.043
Teixeira APC, Santos EM, Vieira AFP, Lago RM (2013) Use of chrysotile to produce highly dispersed K-doped MgO catalyst for biodiesel synthesis. Chem Eng J 232:104–110. https://doi.org/10.1016/j.cej.2013.07.065
Wang W, Qiao X, Chen J, Tan F (2008) Preparation and characterization of Ti-doped MgO nanopowders by a modified coprecipitation method. J Alloys Compd 461:542–546. https://doi.org/10.1016/j.jallcom.2007.07.046
Xue B, Luo J, Zhang F, Fang Z (2014) Biodiesel production from soybean and Jatropha oils by magnetic CaFe2O4-Ca2Fe2O5-based catalyst. Energy 68:584–591. https://doi.org/10.1016/j.energy.2014.02.082
Zabeti M, Wan Daud WMA, Aroua MK (2009) Activity of solid catalysts for biodiesel production: a review. Fuel Process Technol 90:770–777. https://doi.org/10.1016/j.fuproc.2009.03.010
Zhang X, Huang W (2011) Biodiesel fuel production through transesterification of Chinese tallow kernel oil using KNO3/MgO catalyst. Procedia Environ Sci 11:757–762. https://doi.org/10.1016/j.proenv.2011.12.117
Zhou J, Yang S, Yu J (2011) Facile fabrication of mesoporous MgO microspheres and their enhanced adsorption performance for phosphate from aqueous solutions. Colloids Surf A Physicochem Eng Asp 379:102–108. https://doi.org/10.1016/j.colsurfa.2010.11.050
Acknowledgements
All the authors sincerely thank VIT Chennai Campus, Chennai, Tamilnadu, India, for providing financial assistance through research associateship to the first author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashok, A., Kennedy, L.J., Vijaya, J.J. et al. Optimization of biodiesel production from waste cooking oil by magnesium oxide nanocatalyst synthesized using coprecipitation method. Clean Techn Environ Policy 20, 1219–1231 (2018). https://doi.org/10.1007/s10098-018-1547-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-018-1547-x