Abstract
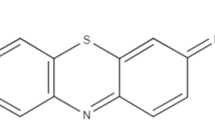
The feasibility of using peanut husk biomass for the removal of Indosol Orange RSN dye was explored during this study. Batch experiments were conducted with native, polyethyleneimine (PEI) treated and Na-alginate immobilized biomass. Different important process parameters like pH, contact time, biosorbent dose, initial dye concentration, and temperature were optimized during batch study. Low pH and low biosorbent dose were found to be the feasible conditions for the maximum biosorption of dye. PEI-treated biomass exhibited maximum biosorption capacity (79.5 mg g−1) for Indosol Orange RSN dye. Pseudo-second-order equation generated the best agreement with experimental data. Different equilibrium isotherm models were applied to the experimental data. Langmuir adsorption isotherm model showed better fitness to the experimental results. Biosorption process was found to be exothermic in nature and thermodynamic study was carried out to check out the feasibility of process. Continuous mode study was performed with native peanut husk biomass to optimize the bed height, flow rate, and initial dye concentration for maximum dye removal. The results indicate that maximum dye removal (8.8 mg L−1) was obtained with 3 cm bed height and 1.8 mL min−1 flow rate by using 70 mg L−1 initial dye concentration. Characterization of biosorbent was carried out by determination of point of zero charge, scanning electron microscopy, and Fourier transform infrared spectroscopy. The findings revealed that peanut husk biomass has a high biosorption potential, and it can be exploited for the treatment of dye containing waste water.







Similar content being viewed by others
References
Abdullah MA, Chiang L, Nadeem M (2009) Comparative evaluation of adsorption kinetics and isotherms of a natural product removal by Amberlite polymeric adsorbents. Chem Eng J 146:370–376
Ahmad AA, Hameed BH (2010) Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J Hazard Mater 175:298–303
Ahmad R, Kumar R (2010) Adsorption studies of hazardous malachite green onto treated ginger waste. J Environ Manag 91:1032–1038
Akar T, Anilan B, Gorgulu A, Akar ST (2009) Assessment of cationic dye biosorption characteristics of untreated and non-conventional biomass: pyracantha coccinea berries. J Hazard Mater 168:1302–1309
Al-Degs YS, Khraisheh MAM, Allen SJ, Ahmad MN (2009) Adsorption characteristics of reactive dyes in columns of activated carbon. J Hazard Mater 165:944–949
Allen SJ, Gan Q, Matthews R, Johnso PA (2005) Mass transfer processes in the adsorption of basic dyes by peanut hulls. Ind Eng Chem Res 44:1942–1949
Al-Qodah Z, Lafi W (2003) Continuous adsorption of acid dyes in fixed beds. J Water Supply 52(3):189–198
Amin NK (2009) Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: adsorption equilibrium and kinetics. J Hazard Mater 165:52–62
Arias F, Sen TK (2009) Removal of zinc metal ion (Zn2+) from its aqueous solution by kaolin clay mineral: a kinetic and equilibrium study. Colloids Surf A 348:100–108
Asgher M, Bhatti HN (2011) Removal of Reactive Blue 19 and Reactive Blue 49 textile dyes by citrus waste biomass from aqueous solution: equilibrium and kinetic study. Can J Chem Eng 9999:1–8
Asgher M, Bhatti HN (2012) Evaluation of thermodynamics and effect of chemical treatments on sorption potential of Citrus waste biomass for removal of anionic dyes from aqueous solutions. Ecol Eng 38:79–85
Bhattacharya AK, Mandal SN, Das SK (2006) Adsorption of Zn(II) from aqueous solution by using different adsorbents. Chem Eng J 123:43–51
Bhattacharyya KG, Gupta SS (2006) Adsorption of Fe(III) from water by natural and acid activated clays: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Adsorption 12:185–204
Bhatti HN, Khalid R, Hanif MA (2009) Dynamic biosorption of Zn(II) and Cu(II) using pretreated Rosa gruss an teplitz (red rose) distillation sludge. Chem Eng J 148:434–443
Bohart GS, Adams EQ (1920) Some aspects of the behaviour of charcoal with respect to chlorine. J Am Chem Soc 42:523–544
Daud NK, Hameed BH (2010) Decolorization of Acid Red 1 by Fenton-like process using rice husk ash-based catalyst. J Hazard Mater 176:938–944
Deng S, Ting YP (2007) Removal of As(V) and As(III) from water with a PEI-modified fungal biomass. Water Sci Technol 55:177–185
Doubinin MM, Radushkevich LV (1947) Proceedings of the academy of sciences of the USSR. Phys Chem 55:327–329
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Freundlich HMF (1906) Ober dies adsorption in losungen. J Phys Chem 57:385–470
Fu F, Wang Q, Tang B (2010) Effective degradation of C.I. Acid Red 73 by advanced Fenton process. J Hazard Mater 174:17–22
Gong R, Ding Y, Li M, Yang C, Liu H, Sun Y (2005) Utilization of powdered peanut hull as biosorbent for removal of anionic dyes from aqueous solution. Dyes Pig 64:187–192
Gonzo EE, Gonzo LF (2005) Kinetics of phenol removal from aqueous solution by adsorption onto peanut shell acid activated carbon. Adsorp Sci Technol 23(4):289–302
Gupta VK, Srivastava SK, Mohan D, Sharma S (1997) Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions. Waste Manag 17(8):517–522
Gupta VK, Jain R, Varshney S (2007) Electrochemical removal of the hazardous dye Reactofix Red 3 BFN from industrial effluents. J Colloid Interface Sci 312(2):292–296
Gupta VK, Mittal A, Malviya A, Mittal J (2009) Adsorption of carmoisine A from wastewater using waste materials—Bottom ash and deoiled soya. J Colloid Interface Sci 335(1):24–33
Gupta VK, Rastogi A, Nayak A (2010) Biosorption of nickel onto treated alga (Oedogonium hatei): application of isotherm and kinetic models. J Colloid Interface Sci 342(2):533–539
Gupta VK, Agarwal S, Saleh TA (2011a) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185(1):17–23
Gupta VK, Gupta B, Rastogi A, Agarwal S, Nayak A (2011b) A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—Acid Blue 113. J Hazard Mater 186(1):891–901
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore and solid diffusion kinetics in fixed bed adsorption under constant pattern conditions. IEC Fundam 5:212–223
Hameed BH, El-Khaiar MI (2008) Malachite green adsorption by rattan sawdust: isotherm, kinetic and mechanism modeling. J Hazard Mater 159:574–579
Hameed BH, Krishni RR, Sata SA (2009) A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. J Hazard Mater 162:305–311
Han R, Wang Y, Yu W, Zou W, Shi J, Liu H (2007) Biosorption of methylene blue from aqueous solution by rice husk in a fixed-bed column. J Hazard Mater 141:713–718
Haq I, Bhatti HN, Asgher M (2011) Removal of Solar Red BA textile dye from aqueous solution by low cost barley husk: equilibrium, kinetic and thermodynamic study. Can J Chem Eng 89:593–600
Harkins WD, Jura CJ (1944) Surfaces of solids XIII. A vapor adsorption method for the determination of the area of a solid without the assumption of a molecular area, and the areas occupied by nitrogen and other molecules on the surface of a solid. J Am Chem Soc 66:1366–1373
Helfferic F (1962) Ion exchange. McGraw-Hill, New York
Ho YS, Mckay G, Wase DAJ, Foster CF (2000) Study on the sorption of divalent metal ions onto peat. Adsorp Sci Technol 18:639–650
Hsueh CL, Huang YH, Wang CC, Chen CY (2005) Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere 58:1409–1414
Jain AK, Gupta VK, Bhatnagar A, Suhas (2003) A comparative study of adsorbents prepared from industrial wastes for removal of dyes. Sep Sci Technol 38(2):463–481
Jain R, Gupta VK, Sikarwar S (2010) Adsorption and desorption studies on hazardous dye Naphthol Yellow S. J Hazard Mater 182:749–756
Juang LC, Lee CK, Wang CC, Hung SH, Lyu MD (2008) Adsorptive removal of Acid Red 1 from aqueous solution with surfactant modified titanate nanotubes. Environ Eng Sci 25:519–528
Karthikeyan S, Balasubramanian R, Iyer CSP (2007) Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresour Technol 98:452–455
Khattri SD, Singh MK (1999) Adsorption of basic dyes from aqueous solution by natural adsorbents. Ind J Chem Technol 6:112–116
Ko DCK, Porter JF, McKay G (2000) Optimised correlations for the fixedbed adsorption of metal ions on bone char. Chem Eng Sci 55:5819–5829
Kose TE (2008) Agricultural residue anion exchanger for removal of dyestuffs from wastewater using full factorial design. Desalination 222:323–330
Kumar PS, Ramalingam S, Senthamarai C, Niranjanaa M, Vijayalakshmi P, Sivanesan S (2010) Adsorption of dye from aqueous solution by cashew nut shell: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 261(1–2):52–60
Kundu S, Gupta AK (2006) Adsorptive removal of As (III) from aqueous solution using iron oxide coated cement (IOCC): evaluation of kinetic, equilibrium and thermodynamic models. Sep Purif Technol 51:165–172
Lagergren S (1898) Zur theorie der sogenannten adsorption gelster stoffe. KungligaSvenska Vetenskapsakademiens Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lezehari M, Baudu M, Bouras O, Basly JP (2012) Fixed-bed column studies of pentachlorophenol removal by use of alginate-encapsulated pillared clay microbeads. J Colloid Interface Sci 379(1):101–106
Li W, Yue Q, Tu P, Ma Z, Gao B, Li J, Xu X (2011) Adsorption characteristics of dyes in columns of activated carbon prepared from paper mill sewage sludge. Chem Eng J 178:197–203
Lu WZ, Leung YT (2003) A preliminary study on potential of developing shower/laundry wastewater reclamation and reuse system. Chemosphere 52:1451–1459
Lu X, Liu L, Liu R, Chen J (2010) Textile wastewater reuse as an alternative water source for dyeing and finishing processes: a case study. Desalination 258:229–232
Mall ID, Shrivastava VC, Kumar GVA, Mishra IM (2006) Characterization and utilization of mesoporous fertilizer plant waste carbon for adsorptive removal of dyes from aqueous solution. Colloids Surf A 278(1–3):175–187
McKay G, Ho YS (1999) The sorption of lead(II) on peat. Water Res 33:578–584
Mittal A, Gupta VK, Malviya A, Mittal J (2008) Process development for the batch and bulk removal and recovery of a hazardous, water-soluble azo dye (Metanil Yellow) by adsorption over waste materials (bottom ash and de-oiled soya). J Hazard Mater 151(2–3):821–832
Mittal A, Kaur D, Malviya A, Mittal J, Gupta VK (2009a) Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents. J Colloid Interface Sci 337(2):345–354
Mittal A, Mittal J, Malviya A, Gupta VK (2009b) Adsorptive removal of hazardous anionic dye Congo red from wastewater using waste materials and recovery by desorption. J Colloid Interface Sci 340:16–26
Mittal A, Mittal J, Malviya A, Gupta VK (2010a) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interface Sci 344(2):497–507
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010b) Adsorption of hazardous dye crystal violet from wastewater by waste materials. J Colloid Interface Sci 343(2):463–473
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010c) Decoloration treatment of a hazardous triarylmethane dye, Light Green SF (Yellowish) by waste material adsorbents. J Colloid Interface Sci 342:518–527
Mohan SV, Mohan SK, Karthikeyan J (2000) Adsorption mechanism of acid-azo dye from aqueous solution on to coal/coal based sorbents and activated carbon: a mechanist study. In: Jayarama Reddy S (ed) Analytical techniques in monitoring the environment, 97th edn., pp 97–103
Mukhopadhyay M, Noronha SB, Suraishkumar GK (2008) Copper biosorption in a column of pretreated Aspergillus niger biomass. Chem Eng J 144:386–390
Önal Y, Akmil-Basar C, Eren D, SarIcI-Özdemir Ç, Depci T (2006) Adsorption kinetics of malachite green onto activated carbon prepared from Tunçbilek lignite. J Hazard Mater 128:150–157
Onyango MS, Kojima Y, Aoyi O, Bernardo EC, Matsuda H (2004) Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J Colloid Interface Sci 279:341–350
Özcan A, Ömeroglu Ç, Erdogan Y, Özcan AS (2007) Modification of bentonite with a cationic surfactant: an adsorption study of textile dye Reactive Blue 19. J Hazard Mater 140:173–179
Padmesh TVN, Vijayaraghavan K, Sekaran G, Velan M (2005) Batch and column studies on biosorption of acid dyes on fresh water macro alga Azolla filiculoides. J Hazard Mater 125:121–129
Patil AK, Shrivastava VS (2010) Alternanthera bettzichiana plant powder as low cost adsorbent for removal of Congo red from aqueous solution. Int J Chem Tech Res 2:842–850
Periasamy K, Namasivayam C (1996) Removal of copper (II) by adsorption onto peanut hull carbon from water and copper plating industry wastewater. Chemosphere 32(4):769–789
Poghossian AA (1997) Determination of the pHpzc of insulators surface from capacitance–voltage characteristics of MIS and EIS structures. Sens Actuator B 44:551
Reddy MCS, Sivaramakrishna L, Reddy AV (2012) The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J Hazard Mater 203–204:118–127
Ricordel S, Taha S, Cisse I, Dorang G (2001) Heavy metals removal by adsorption onto peanut husks carbon: characterization, kinetic study and modeling. Sep Purif Technol 24:389–401
Sadaf S, Bhatti HN (2011) Biosorption of Foron turquoise SBLN using mixed biomass of white rot fungi from synthetic effluents. Afr J Biotechnol 10(62):13548–13554
Sadaf S, Bhatti HN, Ali S, Rehman K (2013) Removal of Indosol Turquoise FBL dye from aqueous solution by bagasse, a low cost agricultural waste: batch and column study. Desalin Water Treat. doi:10.1080/19443994.2013.780985
Saeed A, Sharif M, Iqbal M (2010) Application potential of grapefruit peel as dye sorbent: kinetics, equilibrium and mechanism of crystal violet adsorption. J Hazard Mater 179:564–572
Safa Y, Bhatti HN (2011a) Adsorptive removal of direct textile dyes by low cost agricultural waste: application of factorial design analysis. Chem Eng J 167:35–41
Safa Y, Bhatti HN (2011b) Adsorptive removal of direct dyes by low cost rice husk: effect of treatments and modifications. Afr J Biotechnol 10(16):3128–3142
Safa Y, Bhatti HN, Bhatti IA, Asgher M (2011) Removal of Direct Red-31 and Direct Orange-26 by low cost rice husk: influence of immobilisation and pretreatments. Can J Chem Eng 9999:1–12
Salleh MAM, Mahmoud DK, Karim WA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280(1–3):1–13
Savova D, Petrov N, Yardim MF, Ekinci E, Budinova T, Razvigorova M, Minkova V (2003) The influence of the texture and surface properties of carbon adsorbents obtained from biomass products on the adsorption of manganese ions from aqueous solution. Carbon 41:1897–1903
Song J, Zou W, Bian Y, Su F, Han R (2011) Adsorption characteristics of methylene blue by peanut husk in batch and column modes. Desalination 265:119–125
Tahir SS, Rauf N (2006) Removal of cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 63:1842–1848
Taty-Costodes VC, Fauduet H, Porte C, Ho YS (2005) Removal of lead (II) ions from synthetic and real effluents using immobilized Pinus svlvestris sawdust: adsorption on a fixed column. J Hazard Mater 123:135–144
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physiochem USSR 12:217–222
Thomas HC (1944) Heterogeneous ion exchange in a flowing system. J Am Chem Soc 66:1466–1664
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo red adsorption by clay materials. Chem Eng J 148:354–364
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–59
Zhang L-S, Wu W-Z, Wang J-J (2007) Immobilization of activated sludge using improved polyvinyl alcohol (PVA) gel. J Environ Sci 19:1293–1297
Acknowledgments
The authors are thankful to Higher Education Commission (HEC) of Pakistan for financial assistance under Project No. 20-159/R7D/09/1841 and Indigenous PhD Fellowship Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadaf, S., Bhatti, H.N. Evaluation of peanut husk as a novel, low cost biosorbent for the removal of Indosol Orange RSN dye from aqueous solutions: batch and fixed bed studies. Clean Techn Environ Policy 16, 527–544 (2014). https://doi.org/10.1007/s10098-013-0653-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-013-0653-z