Abstract
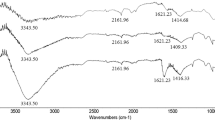
This study relates to optimization of process conditions for sulfur impregnation process on to a virgin steam-activated commercial carbon, with the objective of maximizing the mercuric chloride adsorption. Process optimization was performed using response surface methodology adopting Box–Behnken design. The chosen key process parameters being impregnation temperature (IT), carbon to sulfur ratio (CSR), and impregnation time (ITE), with lower and upper bounds for process conditions being 250–600 °C IT, 0.5–4 CSR, and 15–45 min ITE. The optimum conditions were identified to be an IT of 544 °C, CSR of 0.53 and ITE of 43 min with the HgCl2 adsorption capacity of 85 mg/g. The optimized process conditions were further ensured with repeat runs with reproducible results within acceptable variation. Langmuir and Freundlich equilibrium adsorption isotherm models fitted well with the experimental data. The maximum monolayer adsorption capacity was found to be 294 mg/g which was quite high as compared with the maximum reported in literature. Further, the SEM–EDX analysis evidence the higher amount of sulfur and uniform distribution of sulfur over the surface of activated carbon.





Similar content being viewed by others
References
Anoop Krishnan K, Anirudhan TS (2002) Removal of mercury(II) from aqueous solutions and chlor-alkali industry effluent by steam activated and sulphurised activated carbons prepared from bagasse pith: kinetics and equilibrium studies. J Hazardous Mater 92(2):161–183
Budinova T et al (2006) Characterization and application of activated carbon produced by H3PO4 and water vapor activation. Fuel Process Technol 87(10):899–905
Granite EJ, Pennline HW, Hargis RA (2000) Novel sorbents for mercury removal from flue gas. Ind Eng Chem Res 39(4):1020–1029
Hameed BH, Tan IAW, Ahmad AL (2009) Preparation of oil palm empty fruit bunch-based activated carbon for removal of 2,4,6-trichlorophenol: optimization using response surface methodology. J Hazard Mater 164(2–3):1316–1324
Hsi H-C et al (2001) Effects of sulfur impregnation temperature on the properties and mercury adsorption capacities of activated carbon fibers (ACFs). Environ Sci Technol 35(13):2785–2791
Huiping L et al (2007) Technologic parameter optimization of gas quenching process using response surface method. Comput Mater Sci 38(4):561–570
Karatza D et al (2000) Study of mercury absorption and desorption on sulfur impregnated carbon. Exp Thermal Fluid Sci 21(1–3):150–155
Korpiel JA, Vidic RD (1997) Effect of sulfur impregnation method on activated carbon uptake of gas-phase mercury. Environ Sci Technol 31(8):2319–2325
Li YH, Lee CW, Gullett BK (2003) Importance of activated carbon’s oxygen surface functional groups on elemental mercury adsorption. Fuel 82(4):451–457
Liu W, Vidić RD, Brown TD (1998) Optimization of sulfur impregnation protocol for fixed-bed application of activated carbon-based sorbents for gas-phase mercury removal. Environ Sci Technol 32(4):531–538
Liu W, Vidic RD, Brown TD (1999) Optimization of high temperature sulfur impregnation on activated carbon for permanent sequestration of elemental mercury vapors. Environ Sci Technol 34(3):483–488
Madhava Rao M et al (2006) Removal of copper and cadmium from the aqueous solutions by activated carbon derived from Ceiba pentandra hulls. J Hazard Mater 129(1–3):123–129
Nabais JV et al (2006) Mercury removal from aqueous solution and flue gas by adsorption on activated carbon fibres. Appl Surf Sci 252(17):6046–6052
Namasivayam C, Kadirvelu K (1999) Uptake of mercury (II) from wastewater by activated carbon from an unwanted agricultural solid by-product: coirpith. Carbon 37(1):79–84
Pavlish JH et al (2004) Application of sorbents for mercury control for utilities burning lignite coal. Fuel Process Technol 85(6–7):563–576
ShamsiJazeyi H, Kaghazchi T (2010) Investigation of nitric acid treatment of activated carbon for enhanced aqueous mercury removal. J Ind Eng Chem 16(5):852–858
Sinha RK, Walker PL (1972) Removal of mercury by sulfurized carbons. Carbon 10(6):754–756
Wajima T, Sugawara K (2011) Adsorption behaviors of mercury from aqueous solution using sulfur-impregnated adsorbent developed from coal. Fuel Process Technol 92(7):1322–1327
Yardim MF et al (2003) Removal of mercury (II) from aqueous solution by activated carbon obtained from furfural. Chemosphere 52(5):835–841
Zabihi M, Haghighi Asl A, Ahmadpour A (2010) Studies on adsorption of mercury from aqueous solution on activated carbons prepared from walnut shell. Hazardous Mater 174(1-3):251–256
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rashid, K., Suresh Kumar Reddy, K., Shoaibi, A.A. et al. Sulfur-impregnated porous carbon for removal of mercuric chloride: optimization using RSM. Clean Techn Environ Policy 15, 1041–1048 (2013). https://doi.org/10.1007/s10098-012-0564-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-012-0564-4