Abstract
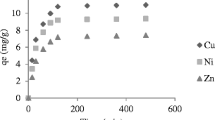
Sludge residues, an industrial waste material for the removal of cadmium (Cd2+), copper (Cu2+), lead (Pb2+), and zinc (Zn2+) from aqueous solutions were investigated using batch method. Batch mode experiments were carried out as a function of solution pH, adsorbent dosage, initial concentration, and contact time. The results indicated that the adsorbent showed good sorption potential and maximum metal removal was observed at pH ≥ 3. Within 120 min of operation, about 63.7, 95.2, 99.9, and 88.2% of Cd2+, Cu2+, Pb2+, and Zn2+ ions were removed from the solutions, respectively. Sorption curves were well fitted to the Langmuir and Freundlich models. The adsorption capacities for Cd2+, Cu2+, Pb2+, and Zn2+ ions at optimum conditions were 121.2, 1067.8, 566.4, and 534.2 mg g−1, respectively. The kinetics of Cd2+, Cu2+, Pb2+, and Zn2+ adsorption from aqueous solutions was analyzed by fitting the experimental data to pseudo-first- and pseudo-second-order kinetic models. However, the pseudo-first-order kinetics model provided much better R 2 values and the rate constant was found to be 0.001 min−1 for Cd2+, Cu2+, Pb2+, and Zn2+ ions. The results revealed that sludge residues can adsorb considerable amount of Cd2+, Cu2+, Pb2+, and Zn2+ ions and it could be an economical method for the removal of these ions from aqueous systems.








Similar content being viewed by others
References
Acar FN, Eren Z (2006) Removal of Cu ions by activated poplar sawdust (Samsun Clone) from aqueous solutions. J Hazard Mater 137:909–914
Aklil A, Mouflih M, Sebti S (2004) Removal of heavy metal ions from water by using calcined phosphate as a new adsorbent. J Hazard Mater A112:183–190
Allison JD, Brown DS, Novo-Gradac KJ (1991) MINTEQA2/PRODEFA2, A geochemical assessment model for environmental systems: version 3.0 Users manual. US Environmental Protection Agency, Athens. (EPA/600/3-91/021)
Altundogan HS, Tumen F (2001) Removal of phosphates from aqueous solutions by using bauxite. I. Effect of pH on the adsorption of various phosphates. J Chem Technol Biotechnol 77:77–85
Alyüz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J Hazard Mater 167:482–488
Argun MA, Dursun S, Ozdemir C, Karatas M (2007) Heavy metal adsorption by modified oak sawdust: thermodynamics and kinetics. J Hazard Mater 141:77–85
Arias M, Barral MT, Mejuto JC (2002) Enhancement of copper and cadmium adsorption on kaolin by the presence of humic acids. Chemosphere 48:1081–1088
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243
Bayat B (2002) Comparative study of adsorption properties of turkish fly ashes. II. The case of chromium(VI) and cadmium(II). J Hazard Mater 95:275–290
Beukes JP, Giesekke EW, Elliott W (2000) Nickel retention by goethite and hematite. Miner Eng 13:1573–1579
Blowes DW, Jambor JL (1990) The pore-water geochemistry and the mineralogy of the vadose zone of sulphide tailings, Waite Amulet, Quebec, Canada. Appl Geochem 5:327–346
Brady PV, Papenguth HW, Kelly JW (1999) Metal sorption to dolomite surfaces. Appl Geochem 14:569–579
Cardenas G, Orlando P, Edelio T (2001) Synthesis and applications of chiston mercaptanes as heavy metal retention agent. Int J Biol Macromol 28:167–174
Chen Q, Hills CD, Yuan M, Liu H, Tyrer M (2008) Characterization of carbonated tricalcium silicate and its sorption capacity for heavy metals: a micron scale composite adsorbent of active silicate gel and calcite. J Hazard Mater 153:775–783
Collins RC, Ragnarsdottir KV, Sherman DM (1999) Effect of inorganic and organic ligands on the mechanism of cadmium sorption to goethite. Geochim Cosmochim Acta 63:2989–3002
Davis JA, Fuller CC, Cook AD (1987) A model for trace element sorption processes at the calcite surface: adsorption of Cd2+ and subsequent solid-solution formation. Geochim Cosmochim Acta 51:1477–1490
El-Kamash AM, Zaki AA, El Geleel MA (2005) Modeling batch kinetics and thermodynamics of zinc and cadmium ions removal from waste solutions using synthetic zeolite A. J Hazard Mater B127:211–220
Elzinga EJ, Reeder RJ (2002) X-ray absorption spectroscopy study of Cu2+ and Zn2+ adsorption complexes at the calcite surface: implications for site-specific metal incorporation preferences during calcite crystal growth. Geochim Cosmochim Acta 66:3943–3954
Elzinga EJ, Rouff AA, Reeder RJ (2006) The long-term fate of Cu2+, Zn2+, and Pb2+ adsorption complexes at the calcite surface: an X-ray absorption spectroscopy study. Geochim Cosmochim Acta 70:2715–2725
Erdem M, Zverdi O (2005) Lead adsorption from aqueous solution onto siderite. Sep Purif Technol 42:259–264
Erdem M, Altundogan HS, Tumen F (2004) Removal of hexavalent chromium by using heat-activated bauxite. Miner Eng 17:1045–1052
Ettler V, Zelena O, Mihaljevic M, Sebek O, Strnad L, Coufal P, Bezdicka P (2006) Removal of trace elements from landfill leachate by calcite precipitation. J Geochem Explor 88:28–31
Fuller CC, Davis JA (1987) Processes and kinetics of Cd2+ adsorption by a calcareous aquifer sand. Geochim Cosmochim Acta 51:1491–1502
Fuller CC, Bargar JR, Davis JA, Piana MJ (2002) Mechanisms of uranium interactions with hydroxyapatite: implications for groundwater remediation. Environ Sci Technol 36:158–165
Garg K, Gupta R, Kumar R, Gupta RK (2004) Adsorption of chromium from aqueous solution on treated sawdust. Bioresour Technol 92:79–81
Go’mez del Rıo JA, Morandoa PJ, Cicerone DS (2004) Natural materials for treatment of industrial effluents: comparative study of the retention of Cd, Zn and Co by calcite and hydroxyapatite. Part I: batch experiments. J Environ Manag 71:169–177
Godelitsas A, Astilleros JM, Hallam K, Harissopoulos S, Putnis A (2004) Interaction of calcium carbonates with lead in aqueous solutions. Environ Sci Technol 37:3351–3360
Gupta G, Torres N (1998) Use of fly ash in reducing toxicity of and heavy metals in wastewater effluent. J Hazard Mater 57:243–248
Hamidi AA, Mohd NN, Kamar SA (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour Technol 99:1578–1583
Ho YS, McKay GA (1998) Comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Trans Inst Chem Eng 76B:332–340
Hui KS, Chao CYH, Kot SC (2005) Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J Hazard Mater 27:89–101
Jang A, Seo Y, Bishop PL (2005) The removal of heavy metals in urban runoff by sorption on mulch. Environ Pollut 133:117–127
Johansson L (1999) Blast furnace slag as phosphorus sorbents—column studies. Sci Total Environ 229:89–97
Kanungo SB, Tripathy SS, Mishra SK, Sahoo Rajeev B (2004) Adsorption of Co2+, Ni2+, Cu2+ and Zn2+ onto amorphous hydrous manganese dioxide from simple (1–1) electrolyte solutions. J Colloid Interf Sci 269:11–21
Komnitsas K, Bartzas G, Paspaliaris BI (2004) Efficiency of limestone and red mud barriers: laboratory column studies. Minerals Eng 17:183–194
Kurniawan TA, Chan GYS, Lo WH, Babel S (2006) Physicochemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118:83–98
Kwon JS, Yun ST, Lee JH, Kim SO, Young Jo H (2010) Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic(III) from aqueous solutions using scoria: kinetics and equilibria of sorption. J Hazard Mater 174:307–313
Lehman RG, Harter RD (1984) Assessment of copper-soil bond strength by desorption kinetics. Soil Sci Soc Am J 48:769
Leyva A, Marrero J, Smichowsky P, Cicerone D (2001) Sorption of antimony onto hydroxyapatite. Environ Sci Technol 35:3669–3675
Lin CY, Yang DH (2002) Removal of pollutants from wastewater by coal bottom ash. J Environ Sci Health A 37:1509–1522
Liu C, Huang M (2003) Kinetics of lead adsorption by iron oxides formed under the influence of citrate. Geochim Cosmochim Acta 67:1045–1054
Lorens RB (1981) Strontium, cadmium, manganese, and cobalt distribution coefficients in calcite as a function of calcite precipitation rate. Geochim Cosmochim Acta 5:553–561
Madhava Rao M, Chandra Rao GP, Seshaiah K, Choudary NV, Wang MS (2008) Activated carbon from Ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions. Waste Manag 28:849–858
Mathialagan T, Viraraghavan T (2002) Adsorption of cadmium from aqueous solutions by perlite. J Hazard Mater 94:291–303
McBride MB (1980) Chemisorption of Cd2+ on calcite surfaces. Soil Sci Am J 44:26–28
Namasivayam K, Kumuthu M (1998) Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresour Technol 64:77–79
Naseem R, Ve Tahir SS (2001) Removal of Pb(II) from aqueous/acidic solutions by using bentonite as an adsorbent. Water Res 35:3982–3986
Ortiz N, Pires MAF, Bressiani JC (2001) Use of steel converter slag as nickel adsorber to wastewater treatment. Waste Manag 21:631–635
Ouki SK, Kavannagh M (1997) Performance of natural zeolites for the treatment of mixed metal-contaminated effluents. Waste Manag Res 15:383–394
Panday KK, Prasad G, Singh VN (1984) Removal of Cr(VI) from aqueous solutions by adsorption on fly ash–wollastonite. J Chem Technol Biotechnol 34:367–374
Pierrard JC, Rimbault J, Aplincourt M (2002) Experimental study and modelling of lead solubility as a function of pH in mixtures of ground waters and cement waters. Water Res 36:879–890
Pollard SJJ, Fowler GD, Sollars CG, Perry R (1992) Low-cost adsorbents for waste and wastewater treatment: a review. Sci Total Environ 116:31–52
Rouff AA, Elzinga EJ, Reeder RJ, Fisher NS (2005) The influence of pH on the kinetics, reversibility and mechanisms of Pb(II) sorption at the calcite–water interface. Geochim Cosmochim Acta 69:5173–5186
Rouff AA, Elzinga EJ, Reeder RJ, Fisher NS (2006) The effect of aging and pH on Pb(II) sorption processes at the calcite-water interface. Environ Sci Technol 40:1792–1798
Saeed A, Akhter MW, Iqbal M (2005) Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent. Sep Purif Technol 45:25–31
Sanchez AG, Ayuso EA (2002) Sorption of Zn, Cd and Cr on calcite: application to purification of industrial wastewater. Miner Eng 15:539–547
Shirvani M, Shariatmadari H, Kalbasi M, Nourbakhsh F, Najafi B (2005) Sorption of cadmium on palygorskite, sepiolite and calcite: Equilibria and organic ligand affected kinetics. Colloid Surf 287:182–190
Singh T, Pant KK (2004) Equilibrium, kinetics and thermodynamic studies for adsorption of As(III) on activated alumina. Sep Purif Technol 36:139–147
Sposito GA (1984) The surface chemistry of soils. Oxford University Press, New York
Sprynskyy M, Buszewski B, Terzyk AP, Namiesnik J (2006) Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J Colloid Interf Sci 304:21–28
Sturchio NC, Chiarello RP, Cheng LW, Lyman PF, Bedzyk MJ, Qian YL, You HD, Yee D, Geissbuhler P, Sorensen LB, Liang Y, Baer DR (1997) Lead adsorption at the calcite–water interface: synchrotron X-ray standing wave and X-ray reflectivity studies. Geochim Cosmochim Acta 61:251–263
Suraj G, Iyer CSP, Lalithambika M (1998) Adsorption of cadmium and copper by modified kaolinites. Appl Clay Sci 13:293–306
Temmam M, Paquette J, Vali H (2000) Mn and Zn incorporation into calcite as a function of chloride aqueous concentration. Geochim Cosmochim Acta 64:2417–2430
Van Herck P, Van der Bruggen B, Vogels G, Van decasteele C (2000) Application of computer modelling to predict the leaching behaviour of heavy metals from MSWI fly ash and comparison with a sequential extraction method. Waste Manag 20:203–210
Wang Y, Reardon EJ (2001) A siderite/limestone reactor to remove arsenic and cadmium from wastewaters. Appl Geochem 16:1241–1249
Wang YM, Chen TC, Yeh KJ, Shue MF (2001) Stabilization of an elevated heavy metal contaminated site. J Hazard Mater 88:63–74
Weng CH, Tsai CZ, Chu SH, Sharma YC (2007) Adsorption characteristics of copper onto spent activated clay. Sep Purif Technol 54:187–197
Wu Z, Gu Z, Wang X, Evans L, Guo H (2003) Effects of organic acids on adsorption of lead onto montmorillonite, goethite and humic acid. Environ Pollut 121:469–475
Xiao F, Howard Huang JCH (2009) Comparison of biosorbents with inorganic sorbents for removing copper (II) from aqueous solutions. J Environ Manag 90:3105–3109
Yabe MJS, Oliveira E (2003) Heavy metals removal in industrial effluents by sequential adsorbent treatment. Adv Environ Res 7:263–272
Yavuz O, Altunkaynak Y, Guzel F (2003) Removal of copper nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res 37:948–952
Zachara JM, Cowan CE, Resch CT (1991) Sorption of divalent metals on calcite. Geochim Cosmochim Acta 55:1549–1562
Zhang H, He PJ, Shao LM, Li XJ (2008a) Leaching behavior of heavy metals from municipal solid waste incineration bottom ash and its geochemical modeling. J Mater Cycles Waste Manag 10:7–13
Zhang Y, Jiang J, Chen M (2008b) MINTEQ modeling for evaluating the leaching behavior of heavy metals in MSWI fly ash. J Environ Sci 20:1398–1402
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merrikhpour, H., Jalali, M. Waste calcite sludge as an adsorbent for the removal of cadmium, copper, lead, and zinc from aqueous solutions. Clean Techn Environ Policy 14, 845–855 (2012). https://doi.org/10.1007/s10098-012-0450-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-012-0450-0