Abstract
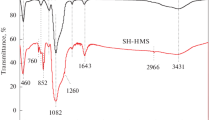
The objective of the study is the development of sorbents where the sorption sites are highly accessible for the capture of mercury from aqueous and vapor streams. Only a small fraction of the equilibrium capacity is utilized for a sorbent in applications involving short residence times (e.g., vapor phase capture of mercury from coal-fired power plant flue gases). So, dynamic capacity rather than equilibrium capacity is more relevant for these kinds of situations. Rapid sorption rates and higher dynamic capacity can be achieved by increasing the accessibility of active sites and decreasing the diffusional resistance to mass transport for the adsorbing species. This requires the use of open structured sorbent materials and attachment of functional groups on the external surface area of supports. The strong interaction of sulfur containing ligands (e.g., thiol) with mercury makes them suitable candidates for immobilization on these types of materials. In this study, inorganic oxide supports like alumina and silica are functionalized with thiol moieties like mercapto silane, cysteine and poly-cysteine for capturing mercury from aqueous and vapor phase. Aqueous phase Hg (II) sorption studies with cysteine/poly-cysteine functionalized silica showed that high dynamic capacity can be achieved by attaching active sites (thiol) on the external area of supports. Vapor phase Hg capture studies with thiol-functionalized mesoporous silica (Hg0 concentration = 3.37 mg/m3 with N2 as the carrier, gas temperature = 70 °C) yielded a capacity of 143 μg Hg/g for the sorbent. Although the sulfur content for the sorbent was low (0.80 wt. %) the molar ratio of Hg captured to sulfur was comparatively high (2.86×10−3) pointing to the high accessibility of sulfur sites.










Similar content being viewed by others
References
Beauvais RA, Alexandratos SD (1998) Polymer-supported reagents for the selective complexation of metal ions: an overview. React Func Polym 36:113–123
Bhattacharyya D, Butterfield DA (2003) New insights into membrane science and technology: polymeric and biofunctional membranes. Elsevier, Amsterdam
Bibby A, Mercier L (2002) Mercury (II) ion adsorption behavior in thiol-functionalized mesoporous silica microspheres. Chem Mater 14:1591–1597
Brown J, Richer R, Mercier L (2000) One-step synthesis of high capacity mesoporous Hg2+ adsorbents by non-ionic surfactant assembly. Microporous Mesoporous Mater 37:41–48
EPA (1997) Mercury study report to congress. Technical Report EPA-452/R-97–003, EPA, Triangle Research Park, North Carolina
Feng X, Fryxell GE, Wang L-Q, Kim AY, Liu J, Kemner KM (1997) Functionalized monolayers on ordered mesoporous supports. Science 276:923–926
Galbreath KC, Zygarlicke CJ (1996) Mercury speciation in coal combustion and gasification flue gases. Environ Sci Technol 30:2421–2426
Granite EJ, Pennline HW, Hargis RA (2000) Novel sorbents for mercury removal from flue gas. Ind Eng Chem Res 39:1020–1029
Gregg SJ, Sing KSW (1967) Adsorption, surface area and porosity. Academic Press, London
Ghorishi SB, Keeney RM, Serre DS, Gullett BK, Jozewicz WS (2002) Development of a Cl-impregnated activated carbon for entrained-flow capture of elemental mercury. Environ Sci Technol 36:4454–4459
Helfferich F (1995) Ion exchange. Dover, New York
Hornback JI (1998) Organic chemistry. Brooks/Cole, California
Hsi H-C, Rood MJ, Rostam-Abadi M, Chen S, Chang R (2001) Mercury adsorption properties of sulfur-impregnated adsorbents. J Environ Eng 128:1080–1089
Iler RK (1979) The chemistry of silica: solubility polymerization colloid and surface properties and biochemistry of silica. Wiley, New York
Kostal J, Mulchandani A, Chen W(2001) Tunable biopolymers for heavy metal removal. Macromolecules 34:2257–2261
Krishnan SV, Gullett BK, Jozewicz W, (1994) Sorption of elemental mercury by activated carbons. Environ Sci Technol 28:1506–1512
Liu W, Vidic RD, Brown TD (2000) Impact of flue gas conditions on mercury uptake by sulfur-impregnated activated carbon. Environ Sci Technol 34:154–159
Marcus Y, SenGupta AK, Marinsky JA (2002) Ion exchange and solvent extraction, vol 15. Dekker, New York
Martinez RF, Rucandio MI (2003) Study of extraction conditions for the quantitative determination of Hg bound to sulfide in soils from Almaden (Spain). Anal Bioanal Chem 375:1089–1096
Martell AE, Smith RM, Motekaitis RM (1995) NIST critically selected stability constants of metal complexes. NIST Standard Reference Database 46 Version 20, NIST Standard Reference Data, Gaithersburg, Maryland
Mercier L, Pinnavaia TJ (1998a) Heavy metal ion adsorbents formed by the grafting of a thiol functionality to mesoporous silica molecular sieves: factors affecting Hg (II) uptake Environ Sci Technol 32:2749–2754
Mercier L, Pinnavaia TJ (1998b) A functionalized porous clay heterostructure for heavy metal ion (Hg2+) trapping. Microporous Mesoporous Mater 20:101–106
Miller TC, Kwak E-S, Howard ME, Bout DAV, Holcombe JA (2001) An in situ study of metal complexation by an immobilized synthetic biopolymer using tapping mode cell atomic force microscopy. Anal Chem 73:4087–4095
Nam KH, Gomez-Salazar S, Tavlarides LL (2003) Mercury (II) sorption from waste waters using a thiol functional adsorbent. Ind Eng Chem Res 42:1955–1964
Pitoniak E, Wu C-Y, Londeree D, Mazyck D, Bonzongo J-C, Powers K, Sigmund W (2003) Nanostructured silica-gel doped with TiO2 for mercury vapor control. J Nanoparticle Res 5:281–292
Pitzer KS (1991) Activity coefficients in electrolyte systems 2nd edn. CRC, Boca Raton, Florida
Ritchie SMC, Kissick KE, Bachas LG, Sikdar SK, Parikh C, Bhattacharyya D (2001) Polycysteine and other poly amino acid functionalized microfiltration membranes for heavy metal capture. Environ Sci Technol 35:3252–3258
Ruthven DM (1984) Principles of adsorption and adsorption processes. Wiley, New York, pp 180–183
SenGupta A K (2002) Environmental separation of heavy metals: engineering processes. Lewis, Boca Raton, Florida
Schmidt-Winkel P, Lukens WW, Zhao D, Yang P, Chemelka BF, Stucky GD (1999) Mesocellular siliceous foams with uniformly sized cells and windows. J Am Chem Soc 121:254–255
Shendrikar A, Damie A, Gutkneckt WF (1984) Technical report, EPA-600/7–84–089, US EPA, Research Triangle Park, North Carolina
Suzuki M (1990) Adsorption engineering. In: Chemical engineering monographs, vol 25. Elsevier, Amsterdam, pp 97–99
Vidic RD, Siler DP (2001) Vapor-phase elemental mercury adsorption by activated carbon impregnated with chloride and chelating agents. Carbon 39:3–14
Walcarius A, Etienne M, Bessiere J (2002) Rate of access to the binding sites in organically modified silicates. 1. Amorphous silica gels grafted with amine or thiol groups. Chem Mater 14:2757–2766
Acknowledgments
The project has been funded by the US EPA and their support is highly appreciated. Thanks are also due to Vasilie Smuleac for his help in functionalizing the alumina membranes. The authors are thankful to Tricia Coakely at the Environmental Research and Training Laboratory (ERTL), University of Kentucky, Lexington for her help in aqueous Hg analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makkuni, A., Bachas, L.G., Varma, R.S. et al. Aqueous and vapor phase mercury sorption by inorganic oxide materials functionalized with thiols and poly-thiols. Clean Techn Environ Policy 7, 87–96 (2005). https://doi.org/10.1007/s10098-004-0260-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-004-0260-0