Abstract
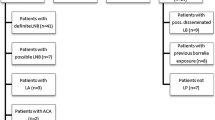
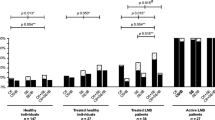
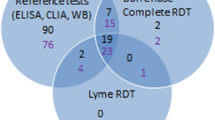
The performance of 11 commercially available enzyme immunoassays (EIA) and four Western blot (WB) tests for the detection of IgM and IgG antibodies against Borrelia burgdorferi were compared. A total of 229 serum specimens were used: 26 from patients with early Lyme borreliosis, 13 from patients with late Lyme borreliosis, 62 from healthy controls and 128 from patients with disorders clinically mimicking Lyme borreliosis and/or known to cause cross-reactivity in Lyme borreliosis serological tests (patient control group). In specimens from patients with early Lyme borreliosis, the sensitivity of the individual tests ranged from 35 to 81% for detection of IgM. In late Lyme borreliosis, sensitivity of the tests ranged from 46 to 92%. In healthy controls the specificity of the tests ranged from 89 to 100% and from 82 to 97% for IgM and IgG tests, respectively. In the patient control group, specificity of the tests ranged from 75 to 90% for IgM and from 84 to 100% for IgG tests. The Behring (Germany) and Genzyme Virotech (Germany) IgM EIA tests showed the best performance in detecting early Lyme borreliosis. For the detection of late Lyme borreliosis, the Dako (Denmark) IgG test was the best despite its low sensitivity. The maximum sensitivity of Western blotting for detecting IgM in patients with early Lyme borreliosis and IgG in patients with late Lyme borreliosis was 50 and 46%, respectively. The use of an EIA-WB two-test protocol improved the specificity and positive predictive values of the EIA results but caused a significant loss in sensitivity. Patients with Epstein-Barr virus or cytomegalovirus infection who had a positive reaction in the IgM EIA could not be discriminated from patients with early Lyme borreliosis with the help of Western blotting. Hence, positive and negative predictive values in combination with sensitivity and specificity values indicated that the exclusion of these infections was more relevant than the confirmation of a positive IgM EIA with Western blot.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goossens, H., van den Bogaard, A. & Nohlmans, M. Evaluation of Fifteen Commercially Available Serological Tests for Diagnosis of Lyme Borreliosis. EJCMID 18, 551–560 (1999). https://doi.org/10.1007/s100960050347
Issue Date:
DOI: https://doi.org/10.1007/s100960050347