Abstract
Objectives
This study aimed to determine the in vitro efficacy of cefiderocol in carbapenem-resistant Acinetobacter baumannii (CRAB) isolates and evaluate the disk-diffusion (DD) method as an alternative method to broth-microdilution (BMD).
Methods
Totally 89 CRAB isolates were included. Cluster analysis was determined by Pulsed-Field Gel Electrophoresis (PFGE). Resistance genes; blaOXA−51, blaOXA−23, blaOXA−24, blaOXA−58,blaPER−1, blaNDM, blaIMP and mcr-1 were screened. Cefiderocol susceptibility testing was performed by both DD and BMD. Interpretation was made according to EUCAST and CLSI. Categorical agreement (CA), minor errors (mEs), major errors (MEs), and very major errors (VMEs) were determined.
Results
PFGE revealed 5 distinct pulsotypes; 86 of the isolates were extensively drug-resistant (XDR). All the isolates were negative for blaNDM, blaIMP, mcr-1, while positive for blaOXA−58 and blaOXA51. blaPER−1 was positive for 33.7%; blaOXA−23 for 74.2%; blaOXA−24 for 12.3%. According to CLSI, the MEs rate was 1.85%, mEs was 7.86% and there were no VMEs. According to EUCAST, MEs rate was 3.70%, there were no mEs and VMEs. CA was 91% for CLSI and 97.8% for EUCAST. MICs of cefiderocol against A. baumannii isolates ranged from 0.06 to > 128 mg/L, with MIC50 and MIC90 values of 0.5 and > 128 mg/L, respectively.
Conclusions
Cefiderocol susceptibility was 60.7% in CRAB isolates. MIC50, MIC90 of blaPER−1 positive and blaPER−1 negative groups were > 128/>128 and 0.25/>128 mg/L. A correlation between the presence of blaPER−1 and cefiderocol resistance was observed (p < 0.0001). Among colistin-resistant isolates, the presence of blaPER−1 was 47.1% and 75% of them were resistant to cefiderocol respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance (AMR) is one of the most serious worldwide public health threats that poses significant challenges to healthcare systems including mortality, morbidity, and healthcare costs [1]. It was estimated that about 5 million deaths were associated with bacterial AMR in 2019 [2]. World Health Organization (WHO) published a list of antibiotic-resistant priority bacterial pathogens that pose the greatest threat to human health, in which carbapenem-resistant A. baumannii (CRAB) was declared as “critical” [3]. CRAB has emerged as a leading cause of healthcare-associated infections, such as pneumonia, bloodstream infections, and urinary tract infections particularly in immune-compromised individuals. A key aspect of A. baumannii is the propensity to develop rapid resistance [4]. According to The Central Asian and European Surveillance of Antimicrobial Resistance network [1], the carbapenem resistance rate of A. baumannii exceeded 90% in some countries such as Turkiye, Serbia, Belarus, and Montenegro. Carbapenem resistance in A. baumannii is mediated by multiple different mechanisms but mostly by acquisition of Ambler class D enzymes, in particular, OXA-23, and OXA-24 [5, 6]. Other carbapenemases, such as OXA-58, NDM, KPC, and IMP have also been reported [6, 7]. Also, extended-spectrum β-lactamases (ESBL) including PER-type which efficiently hydrolyzes penicillins and cephalosporins [8], contribute the emergence of widespread multi-drug-resistant (MDR) strains of A. baumannii. This situation has forced clinicians to use colistin as one of the last therapeutic options in the fight against such infections [9, 10]. According to WHO’s 2020 Global Antimicrobial Resistance Surveillance System data, the rate of colistin resistance worldwide reaches 37% in some countries such as Lithuania. In a study conducted in Turkiye, A. baumannii was reported as the most common isolate (25.1%) of bacterial lower respiratory tract (LRT) infection and the most effective antibiotic was colistin with a resistance rate of 12.9% [11]. The mechanisms of colistin resistance may be due to chromosomal mutations (such as lpxACD, pmrB and pmrA) or transferable plasmid genes called mcr, which can be spread between bacteria by horizontal transfer [12]. With the emergence of resistant isolates, last resort carbapenem and lipopeptide class antibiotics are no longer effective, highlighting the urgent need for new and effective treatment options.
Cefiderocol is a newly developed siderophore cephalosporin [13]. Like other β-lactam antibiotics, it binds to penicillin-binding proteins (especially PBP-3) and has a bactericidal effect on bacterial cell wall synthesis [14, 15]. What makes it unique is its increased transition to the bacterial periplasmic area by iron transport systems with its siderophore-like property [14, 15]. It owes this feature to the catechol part, which is located in the 3rd position of the side chain in its molecular structure and binds to ferric iron [13, 16]. It shows increased stability against β-lactamases due to the catechol group [14,15,16]. Although various mechanisms including amino acid changes in PBP-3, changes in the transcription levels of iron transport protein-encoding genes such as fiu, feoA, and feoB, and downregulation in the TonB-ExbB-ExbD energy transfer system cause cefiderocol resistance in A. baumannii [17,18,19], it is emphasized that some β-lactamases contribute to cefiderocol resistance [17, 19]. PiuA, a TonB-dependent siderophore receptor, and PirA, a ferric-enterobactin receptor, are very important in cefiderocol uptake [19,20,21]. In various studies, disruptions in the piuA gene (such as a frameshift mutation resulting in stop codon formation (K384fs) and F174Y substitution) or in the fepA gene, an ortholog of the pirA gene, (such as a transposon insertion, P635-ISAba125) caused increases in cefiderocol MICs and resistance [20,21,22,23]. In another study, piuA and pirA gene deletion mutants showed increased MICs against siderophore-drug conjugates compared to the standard strain without the deletion [22]. PER-1 as an Ambler class A ESBL, NDM as an Ambler class B metallo- β-lactamase, and OXA-23, OXA-24, and OXA-58 as Ambler class D carbapenemases have been reported among important β-lactamases to cefiderocol resistance [17, 18, 24].
In this study, it was aimed to evaluate the in vitro efficacy of cefiderocol in CRAB isolates with a various resistance gene profile. Broth-microdilution (BMD) is recommended as the reference standard method for cefiderocol susceptibility testing [25, 26], and iron depleted-cation adjusted Mueller Hinton broth (ID-CAMHB) should be used which is not available as ready-to-use. It was also aimed to evaluate disk-diffusion (DD) as an alternative to the BMD.
Material and method
This study was approved by the Baskent University Institutional Review Board (Project no: KA22/392) and supported by Baskent University Research Fund.
Bacterial isolates
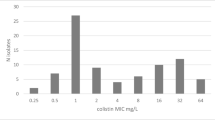
A total of 89 CRAB isolates from the culture collection of Medical Microbiology Laboratory of Baskent University Medical Faculty collected between January to December 2021 were included in the study. The 32.6% (n = 29) of the collected isolates were obtained from lower respiratory tract samples, 23.6% (n = 21) from blood, 20.2% (n = 18) from urine,13.5% (n = 12) from wound, 5.6% (n = 5) from catheter, and 4.5% (n = 4) from biopsy culture. Only one isolate for each patient was included. Identification and antimicrobial susceptibility testing (AST) were performed by the BD Phoenix 50 (BD Diagnostics, USA) automated bacterial identification and antibiotic susceptibility system. The identification was confirmed by the presence of the chromosomal blaOXA−51 gene by PCR. All isolates were resistant to meropenem and imipenem, resistance was also screened by DD. MICs of colistin were determined by the Thermo Fisher Scientific Sensitivity FRCOL kit (Thermo Fisher Scientific, USA). All the AST results were assessed using EUCAST standards [27].
Cluster analysis
Genetic relations among clinical CRAB isolates were determined by PFGE as previously described [28]. Genomic DNA was treated with lysis solutions and digested with ApaI restriction enzyme (Takara, Japan). Electrophoresis was performed in a PFGE system (CHEF-DR III System; Bio-RAD, USD). The gel images were processed and cluster analysis was performed using BioNumerics software. The images were normalized by using standard molecular markers, and banding patterns were compared. Pulsotype typing was performed by the interpretative criteria of Tenover et al. [29].
Detection of antibiotic resistance genes by PCR
Bacterial DNA was extracted by boiling the bacterial suspension at 95oC for 10 min [30]. After centrifugation, the supernatant was transferred to new tubes. Amplification of OXA genes, blaOXA−51, blaOXA−23, blaOXA−24, and blaOXA−58 were performed as previously described [31] The blaPER−1, blaNDM, blaIMP, and mcr-1 genes were screened as described previously [32,33,34]. The primer sequences and amplicon sizes were given in supplementary material. Confirmed positive strains of A. baumannii carrying blaOXA51 gene [35], K. pneumoniae CDC529 carrying blaNDM−1, K. pneumoniae CDC309 blaIMP, and A. baumannii ATCC19606 were used as positive control strains. The PCR products were analyzed by 2% agarose gel containing EtBr.
Cefiderocol susceptibility detection by Kirby Bauer DD method
Cefiderocol susceptibility testing was performed by using MASTDISCS (30 µg) (Mast Group, UK) and cation-adjusted Mueller Hinton agar (CA-MHA) (Oxoid, UK). Plates were incubated for 18–24 h at 35 ± 2 °C in aerobic conditions. Zone diameters were evaluated in the reflected light and from the back of the plate. Interpretations of zone diameters were made according to both EUCAST and CLSI [27, 36].
Cefiderocol MIC detection by BMD
MIC detection was performed by using ready-to-use broth microdilution plates, ComASP®, available in the range of cefiderocol 0.008-128 mg/L (Liofilchem, Italy) according to the recommendation of the manufacturer. Briefly, a suspension of 0.5 McFarland standard was prepared from fresh bacterial colonies grown in overnight incubation. This prepared suspension was diluted 1:20 in 0.9% NaCl. 0.4 ml of the diluted bacterial suspension was added to the tube containing 3.6 ml of ID-CAMHB supplied in the kit. From this suspension, 100 µl were distributed into each well, containing cefiderocol at different concentrations between 0.008 mg/L to 128 mg/L. The plates were incubated for 16–20 h at 35°±2 °C in aerobic conditions. The first well in which the turbidity first disappeared or the turbidity showed a significant decrease compared to the growth control well due to the “trailing effect” was considered as the MIC value. When the “Trailing effect” was observed, 80% inhibition was accepted as the MIC value [25]. MIC value was evaluated in the reflected light from the front of the sensititre plate. Interpretation was made according to both EUCAST and CLSI criteria [27, 36]. E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality control strains for both methods.
Agreement analysis
By using the BMD as a reference method; categorical agreement (CA), minor errors (mEs), major errors (MEs), and very major errors (VMEs) were determined according to CLSI definitions. Acceptance criteria are based on ≥ 90% CA, ≤ 10% mEs, ≤ 3% MEs, and ≤ 1.5% VMEs [37].
Statistical analysis
Pearson and Likelihood Ratio Chi-square test were used for the comparison of categorical variables between groups, and the significance test of the difference between two proportions was used for the comparison of only one result within two groups. Categorical variables were expressed as f (%). Statistical analysis was performed by using Statistical Package for Social Sciences (SPSS) for Windows Ver25.0. In statistical analysis, the significance level was taken as p < 0.05.
Results
Isolate profile
All isolates were collected between January to December 2021. The 86 of all isolates were XDR, and 3 were MDR. According to routinely performed AST results 92.1% (82/89) were resistant (R) to amikacin, 97.7% (87/89) to gentamicin, ciprofloxacin, and levofloxacin, 98.8% (88/89) to trimethoprim-sulfamethoxazole and 19.1% (17/89) to colistin. For tigecycline MIC distribution; 2/89 (2.2%) isolates MICs were 1 mg/L, 1/89 (1.1%) isolates < 2 mg/L, 10/89 (11.2%) isolates 2 mg/L and 76/89 (85.5%) isolates were > 2 mg/L.
All the isolates were found as negative for blaNDM, blaIMP, mcr-1, and blaOXA−58 while positive for blaOXA51. blaPER−1 was positive for 33.7% (30/89); blaOXA−23 was positive for 74.2% (66/89); blaOXA−24 was positive 12.3% (11/89) of the isolates.
PFGE revealed 5 distinct pulsotypes; one major, two intermediates, and two minors (Supplementary Fig. 1) [29]. The major pulsotype included 59 isolates (66.3%) and 96.8% (n = 56) of them were XDR. There were 14 (15.7%) and 9 (10.1%) isolates in intermediate pulsotypes and minor pulsotypes included 2 (2.3%) and 5 (5.6%) isolates.
Susceptibility of cefiderocol by DD and BMD
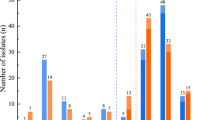
According to CLSI [36], cefiderocol MIC value of ≤ 4 mg/L in A. baumannii is considered as S, 8 mg/L as I, and ≥ 16 mg/L as R; cefiderocol zone diameter of ≥ 15 mm in A. baumannii is considered as susceptible (S), 11–14 mm as intermediate (I), and ≤ 10 mm as R. There is no I category in EUCAST, and resistance of A. baumannii based on PK/PD values while recommending breakpoints as ≤ 2 mg/L S and > 2 mg/L as R; cefiderocol zone diameter ≥ 17 mm as S [27]. MICs of cefiderocol against A. baumannii isolates ranged from 0.06 to > 128 mg/L, with MIC50 and MIC90 values of 0.5 and > 128 mg/L, respectively. The results of cefiderocol susceptibility test according to both EUCAST and CLSI were given in Table 1.
Correlation of DD method with BMD
According to CLSI, 1 R isolate (1.85%) (zone diameter 6 mm) by DD was found as S by BMD (MIC: 0.5 mg/L) which indicates a major error. 7 I isolate (7.86%) (zone diameters 13,14,14,13,13,14,13 mm) by DD were found as R (MICs:16,16,128,32,16,16 and 16 mg/L respectively) by BMD which indicates a minor error. There was no very major error according to CLSI. The 81 (91%) isolates were in categorical agreement.
According to EUCAST, 2 R isolates (3.70%) (zone diameters 15 and 6 mm) by DD were found as S (MICs:2 and 0,5 mg/L) by BMD indicating MEs. There are no VMEs according to EUCAST. Also, there are no mEs due to lack of an I category in EUCAST. Totally 87 (97.8%) isolates were in CA. Table 2 shows the VME, ME, mE, and CA rates of the DD according to CLSI and EUCAST.
In vitro cefiderocol activity according to gene profile
The contribution of blaPER−1, blaOXA−23, and blaOXA−24 genes to cefiderocol resistance was evaluated. Totally 30 (33.7%) isolates were positive for blaPER−1 gene and 25 (83.3%) blaPER−1 positive isolates were resistant to cefiderocol. While 10 (11.2%) of 59 blaPER−1 negative isolates were resistant to cefiderocol. MIC50 and MIC90 values of blaPER−1 positive and blaPER−1 negative isolates were > 128/>128 and 0,25/>128 mg/L respectively. Totally 66 (74.2%) isolates were blaOXA−23 positive and 28 (42.4%) of them were resistant to cefiderocol. While 7 (30.4%) of 23 blaOXA−23 negative isolates were resistant to cefiderocol. The 11 isolates were blaOXA−24 positive and 1 (9.1%) of them was resistant to cefiderocol while 34 (43.6%) blaOXA−24 negative isolates were resistant to cefiderocol. Table 3 shows the MIC distributions of isolates in the presence of resistance genes and Table 4 shows the susceptibility rates in the presence of resistance genes individually and in combinations. Cefiderocol activity of colistin-resistant isolates in the presence and absence of the blaPER−1 gene were also evaluated and shown in Table 5.
Discussion
Cefiderocol is a siderophore cephalosporin that possesses potent activity against carbapenem-resistant and MDR Enterobacterales, and non-fermentative Gram-negative bacilli [14, 16]. Cefiderocol received Food and Drug Administration (FDA) approval for the treatment of urinary tract infections in 2019 and treatment of hospital-acquired pneumonia and ventilator-associated pneumoniae in 2020. Although there are methods such as DD and gradient diffusion to test the susceptibility of cefiderocol, the gold standard method is the BMD [25, 26]. Standard cation-adjusted Mueller Hinton broth is not controlled for iron concentration and iron-depleted cation-adjusted MHB (ID-CAMHB)is required for susceptibility results to be accurate [26, 38]. The CLSI has approved ID-CAMHB and provides instruction to prepare for determining cefiderocol MICs but preparation of this medium is difficult and impractical for the daily laboratory workflow. Also, some gradient diffusion strips were developed but they were reported successful only in P. aureginosa [26]. For all these, DD was also tested for all the isolates included in the study as an alternative method in which standard CA-MHA can be used in the cefiderocol susceptibility testing.
If CLSI is considered in terms of compatibility of DD and BMD; the MEs was 1.85%; mEs was 7.86%, there was no VME while the CA was 91%. Acceptance criteria are based on ≥ 90% CA, ≤ 10% mEs, ≤ 3% MEs, and ≤ 1.5% VME [37]; therefore, the DD seems as a candidate to be a practical and easily applicable alternative to the BMD. But based on CLSI, a total of 7 isolates were in category I according to the DD, while all of these isolates were determined as R according to the BMD; so this result indicates that the results interpreted as I by DD might be R and it would be better to confirm them with the BMD.
According to EUCAST, the MEs was 3.70%, there was no mEs and VME while the CA was 97.8%. With an interpretation according to EUCAST, the DD does not seem as a candidate alternative to the BMD although the CA value was found to be higher than CLSI, the MEs rate (3.7%) was observed above the limit value (3.0%). This might be resulted from the lack of category I in EUCAST which ensures no mEs but causes more MEs.
In a study including CRAB isolates, DD was compared with BMD and HardyDisks with MASTDISCS, and CA was slightly higher in MASTDISCS than in HardyDisks [38]. They also found that for DD, EUCAST breakpoints demonstrated lower susceptibility at 66% compared to CLSI breakpoints with 89% for MASTDISCS. For MASTDISCS, EUCAST breakpoints resulted in the highest CA, however due to the lack of an I category, higher rates of MEs and VMEs were observed. They have indicated that both DD performed poor activity for A. baumannii complex. DD offers a convenient alternative approach to BMD at gram-negative bacteria for cefiderocol AST, with the exception of A. baumannii complex [38]. The results of this study also showed CLSI to be more appropriate than EUCAST as there is no I category [38]. Although DD was not reported as an alternative to BMD in A. baumannii complex in this study; according to our results DD can be an alternative to the BMD if CLSI is used for A. baumannii.
According to another study, 90.9% of 368 carbapenem non-susceptible A. baumannii isolates were found to be susceptible to cefiderocol, while the MIC90 value was 8 mg/L [39]. In another study covering different countries, 76.77% of MDR A. baumannii isolates remained susceptible to cefiderocol, and all cefiderocol resistant isolates were MDR [40]. In the present study, 60.7% of the isolates were S, 39.3% were R, 0% was I according to CLSI criteria; while 59.6% was S, and 40.4% was R according to EUCAST criteria. Higher resistance rates might have resulted from the fact that 96.6% of the isolates were XDR and the high prevalence of blaPER−1 gene (33.7%; p < 0.0001) in isolates may have contributed to cefiderocol resistance. The limitation of the study is that cefiderocol resistance mechanisms were not demonstrated by performing WGS on cefiderocol-susceptible and resistant isolates.
Cefiderocol is known for its high stability to β-lactamases, including ESBLs, AmpC, and carbapenemases [14,15,16]. But it was seen that the presence of blaPER−1 might result higher resistance rate than expected. Even in vitro activity may not reflect the clinical activity. In a study that compared the patient groups treated with the regimens containing cefiderocol to colistin, more microbiological failures were observed -especially in monotherapy- in cefiderocol treated group. All 46 A. baumannii isolates were found as sensitive in vitro according to CLSI and EUCAST. During treatment, microbiological failure was observed in 17.4% (8/46) of patients, and cefiderocol resistance was observed in 8.7% (4/46) [41].
Although many resistance mechanisms have been defined [18, 19, 21, 42], it is stated that the presence of certain β-lactamase genes might be a contributing factor in cefiderocol resistance. Among them, blaPER−1, blaNDM, blaOXA−23, blaOXA−24, blaOXA−58 are considered as very important factors [17, 18, 24, 43]. In our study, the presence of blaPER−1, blaNDM, blaIMP, mcr − 1, blaOXA−23, blaOXA−24, blaOXA−51, and blaOXA−58 genes are screened. The presence of blaPER−1 showed a significant contribution to cefiderocol resistance since 83.3% of blaPER−1 positive isolates were R to cefiderocol (p < 0.0001); while only 11.2% of blaPER−1 negative isolates were R. There was also significant difference between MIC50 values of blaPER−1 positive and blaPER−1 negative groups that MIC50 and MIC90 values were > 128/>128 and 0,25/>128 mg/L respectively. Some other studies also emphasized the role of the blaPER−1 gene in cefiderocol resistance [8, 17, 24, 44].
The cefiderocol resistance rate was higher in blaOXA−23 positive 28/66 isolates (42.4%) than blaOXA−23 negatives 7/23 (30.4%) but the difference was not statistically significant (p > 0.05). The contribution of blaOXA−23 has been emphasized in the co-existence of blaOXA−23 with other β-lactamase genes [18, 43]. The co-existence of blaPER−1 and blaOXA−23 rate was 30.4% in the current study and 81.5% of them were resistant to cefiderocol. The PER-1 plays a pivotal role in cefiderocol resistance. All blaPER−1 positive blaOXA−23 negative isolates (n = 3) were resistant to cefiderocol. Among blaPER−1 negative blaOXA−23 positive isolates (n = 39) the cefiderocol resistance rate was 15.4%. In isolates lacking both genes cefiderocol resistance rate was 20%. So, the presence of the blaOXA−23 gene seems to be only significant in association with the presence of blaPER−1 in cefiderocol resistance in A. baumannii (p < 0.0001). There was no statistically significant difference in cefiderocol resistance between the presence and absence of blaOXA−24 (p > 0.05). The co-existence of blaPER−1 and blaOXA−24 was not detected and among the isolates lacking both genes, cefiderocol resistance rate was significantly lower by 18.8% (p < 0.0001). There seemed to be no blaOXA−24 impact on cefiderocol resistance in A. baumannii isolates.
Colistin is the last resort for the treatment of MDR and XDR A. baumannii infections [9, 10] and the rate of colistin-resistant A. baumannii was observed as 12.9% in a study [11]. The mechanism of colistin resistance is due to chromosomal mutations (such as lpxACD, pmrB, and pmrA) or transferable plasmid genes called mcr-1, which can be spread between bacteria by horizontal transfer [12]. The 19.1% (17/89) isolates were resistant to colistin and none of the A. baumannii isolates was positive for plasmid-mediated mcr-1 resistance. The whole genome sequence of one of our colistin-resistant isolates showed that the colistin resistance resulted from the mutation in pmrA gene as G75A, A120G, G192A, C405T, T450C, C463T and C633T (unpublished data). In another study, 8.2% of A. baumannii isolates were found to be resistant to colistin, and similar to our result, in mcr-1-5 genes were not detected [45]. Cefiderocol was also declared as an alternate agent against colistin-resistant A. baumannii isolates and 64.7% (11/17) of the colistin resistant isolates and 59.7% (43/72) colistin-susceptible isolates were susceptible to cefiderocol which is similar (p > 0.05). The unexpected cefiderocol resistance rate (40.3%) in colistin-susceptible isolates again resulted from the presence of blaPER−1 gene was 30.6% (22/72), and 86.3% (19/22) of these isolates were resistant to cefiderocol. Among colistin-resistant isolates the presence of blaPER−1 was 47.1% (8/17), and 75% (6/8) of them were resistant to cefiderocol while all 9 blaPER−1 negative colistin R isolates were susceptible to cefiderocol (Table 5). The efficacy of cefiderocol against colistin-susceptible or resistant isolates is inversely proportional to the presence of blaPER−1. These results indicate that cefiderocol may be effective in colistin-resistant isolates; suggesting that the efficacy is related to the presence of the blaPER−1 gene rather than the colistin resistance status in A. baumannii.
Data availability
Not applicable.
Code availability
Not applicable.
References
World Health Organization (WHO) (2019) Central Asian and European Surveillance of Antimicrobial Resistance CAESAR Manual Version 3.0
Wagenlehner FME, Dittmar F (2022) Re: global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Eur Urol 82(6):658. https://doi.org/10.1016/j.eururo.2022.08.023
Tacconelli E, Carrara E, Savoldi A (2017) Global Priority List of Antibiotic Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva, Switzerland, pp 1–7
De Oliveira DMP, Forde BM, Kidd TJ et al (2020) Antimicrobial Resistance in ESKAPE pathogens. Clin Microbiol Rev 33(3):1–49. https://doi.org/10.1128/CMR.00181-19
Hamidian M, Nigro S (2019) Emergence, Molecular mechanisms and Global Spread of Carbapenem-Resistant Acinetobacter Baumannii. Microb Genom 5(10):1–13. https://doi.org/10.1099/mgen.0.000306
Lee Y, Ko W, Hsueh P (2023) Geographic patterns of Acinetobacter Baumannii and Carbapenem Resistance in the Asia-Pacific Region: results from the Antimicrobial Testing Leadership and Surveillance (ATLAS) Program, 2012–2019. Int J Infect Dıs 127:48–55. https://doi.org/10.1016/j.ijid.2022.12.010
Poirel L, Nordmann P (2006) Carbapenem Resistance in Acinetobacter Baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12(9):826–836. https://doi.org/10.1111/j.1469-0691.2006.01456.x
Poirel L, Sadek M, Nordmann P (2021) Contribution of PER-Type and NDM-Type β-lactamases to Cefiderocol Resistance in Acinetobacter Baumannii. Antimicrob Agents Chemother 65(10):1–5. https://doi.org/10.1128/AAC.00877-21
Cai Y, Chai D, Wang R (2012) Colistin Resistance of Acinetobacter Baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67(7):1607–1615. https://doi.org/10.1093/jac/dks084
Gordon N, Wareham D (2010) Multidrug Resistant Acinetobacter Baumannii: mechanisms of virulence and resistance. Int J Antimicrob Agents 35(3):219–226. https://doi.org/10.1016/j.ijantimicag.2009.10.024
Guclu AU, Kocak AA, Ok MA et al (2021) Antibacterial Resistance in Lower Respiratory Tract bacterial pathogens: a Multicenter Analysis from Turkey. J Infect Dev Ctries 15(2):254–262. https://doi.org/10.3855/jidc.12599
Nhu NTK, Riordan DW, Nhu TDH et al (2016) The induction and identification of Novel Colistin Resistance mutations in Acinetobacter Baumannii and their implications. Sci Rep 6:1–8. https://doi.org/10.1038/srep28291
Ito A, Nishikawa T, Matsumoto S et al (2016) Siderophore Cephalosporin Cefiderocol utilizes Ferric Iron Transporter Systems for Antibacterial Activity Aginst Pseudomonas Aureginosa. Antimicrob Agents Chemother 60(12):7396–7401. https://doi.org/10.1128/AAC.01405-16
Dobias J, Dénervaud-Tendon V, Poirel L et al (2017) Activity of the Novel Siderophore Cephalosporin Cefiderocol against Multidrug resistant gram-negative pathogens. Eur J Clin Microbiol Infect Dis 36(12):2319–2327. https://doi.org/10.1007/s10096-017-3063-z
Simner PJ, Patel R (2021) Cefiderocol Antimicrobial susceptibility testing considerations: the Achilles’ heel of the trojan horse? J Clin Microbiol 59(1):1–10. https://doi.org/10.1128/JCM.00951-20
Zhanel GG, Golden AR, Zelenitsky S et al (2019) Cefiderocol: a Siderophore Cephalosporin with Activity against Carbapenem resistant and multidrug resistant gram-negative Bacilli. Drugs 79(3):271–289. https://doi.org/10.1007/s40265-019-1055-2
Liu X, Lei T, Yang Y et al (2022) Structural basis of PER-1-Mediated Cefiderocol Resistance and synergistic inhibition of PER-1 by Cefiderocol in Combination with Avibactam or Durlobactam in Acinetobacter Baumannii. Antimicrob Agents Chemother 66(12):1–6. https://doi.org/10.1128/aac.00828-22
Malik S, Kaminsky M, Landman D et al (2020) Cefiderocol Resistance in Acinetobacter Baumannii: roles of β Lactamases, Siderophore receptors, and Penicilin Binding Protein 3. Antimicrob Agents Chemother 64(11):1–4. https://doi.org/10.1128/aac.01221-20
Asrat H, Samaroo-Campbell J, Ata S et al (2023) Contribution of Iron-Transport systems and β-Lactamases to Cefiderocol Resistance in Clinical isolates of Acinetobacter Baumannii Endemic to New York City. Antimicrob Agents Chemother 67(6):1–6. https://doi.org/10.1128/aac.00234-23
Tiseo G, Giordano C, Leonildi A et al (2023) Salvage Therapy with Sulbactam/Durlobactam against Cefiderocol Resistant Acinetobacter Baumannii in a critically ill burn patient: Clinical challenges and molecular characterization. J Antimicrob Chemother 5(3):1–4. https://doi.org/10.1093/jacamr/dlad078
Smoke S, Brophy A, Reveron S et al (2023) Evolution and transmission of Cefiderocol Resistant Acinetobacter Baumannii during an outbreak in the burn Intensive Care Unit. Clin Infect Dis 76(3):1261–1265. https://doi.org/10.1093/cid/ciac647
Moynié L, Luscher A, Rolo D et al (2017) Structure and function of the PiuA and PirA siderophore-drug receptors from Pseudomonas Aeruginosa and Acinetobacter Baumannii. Antimicrob Agents Chemother 61(4):1–14. https://doi.org/10.1128/AAC.02531-16
Yamano Y, Ishibashi N, Kuroiwa M et al (2022) Characterisation of Cefiderocol-Non-susceptible Acinetobacter Baumannii isolates from Taiwan. J Glob Antimicrob Resist 28:120–124. https://doi.org/10.1016/j.jgar.2021.12.017
He Y, Wang Y, Ma X et al (2022) Resistance to Cefiderocol involved expression of PER-1 β-Lactamase and downregulation of Iron Transporter System in Carbapenem Resistant Acinetobacter Baumannii. Infect Drug Resist 15:7177–7187. https://doi.org/10.2147/IDR.S392241
Eucast: Guidance Document on Broth Microdilution Testing of Cefiderocol (2020) https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=408&cHash=c9c0227d00aa8ff6971ace8334a7ee81 Accessed 19 March 2024
Bonnin RA, Emeraud C, Jousset AB et al (2022) Comparison of Disk Diffusion, MIC Test Strip and Broth Microdilution methods for Cefiderocol susceptibility testing on Carbapenem-Resistant Enterobacterales. Clin Microbiol Infect 28(8):1156–1156. https://doi.org/10.1016/j.cmi.2022.04.013
The European Committee on Antimicrobial Susceptibility Testing (2023) Breakpoint tables for interpretation of MICs and zone diameters. Version 13.1, http://www.eucast.org
Gouby A, Carles-Nurit MJ, Bouziges N et al (1992) Use of pulsed-field gel electrophoresis for investigation of hospital outbreaks of Acinetobacter baumannii. J Clin Microbiol 30:1588–1591
Tenover F, Arbeit R, Goering R et al (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J Clin Microbiol 33(9):2233–2239
Guclu AU, Guney M, Sig AK et al (2019) Arising prevalence of OXA-48 producer Escherichia Coli and OXA-48 with NDM Co-producer Klebsiella Pneumoniae strains. Revista Romana De Med De Laborator 27(3):319–326. https://doi.org/10.2478/rrlm-2019-0030
Woodford N, Ellington MJ, Coelho JM et al (2006) Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter Spp. Int J Antimicrob Agents 27(4):351–353. https://doi.org/10.1016/j.ijantimicag.2006.01.004
Poirel L, Walsh TR, Cuvillier V et al (2011) Multiplex PCR for detection of Acquired Carbapenemase genes. Diagn Microbiol Infect Dis 70(1):119–123. https://doi.org/10.1016/j.diagmicrobio.2010.12.002
Liu YY, Wang Y, Walsh TR et al (2016) Emergence of Pplasmid mediated Colistin Resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular Biological Study. Lancet Infect Dis 16(2):161–168. https://doi.org/10.1016/S1473-3099(15)00424-7
Strateva T, Ouzounova-Raykova V, Markova B et al (2007) Problematic clinical isolates of Pseudomonas Aeruginosa from the University hospitals in Sofia, Bulgaria: current status of Antimicrobial Resistance and Prevailing Resistance mechanisms. J Med Microbiol 56(7):956–963. https://doi.org/10.1099/jmm.0.46986-0
Guclu AU, Gozen A (2020) Genetic diversity of OXA-like genes in Multidrug-Resistant Acinetobacter baumannii strains from ICUs. Clin Lab 66(10). https://doi.org/10.7754/Clin.Lab.2020.200135
Lewis IIJS (2023) M100Ed33: Performance Standards for Antimicrobial Susceptibility Testing, 33rd Edition. Malvern, Pennsylvania
Mann LM, Shortridge D (2015) M52Ed1: verify commercial Microbial. ID & AST Systems. Malvern, Pennsylvania
Morris CP, Bergman Y, Tekle T et al (2020) Cefiderocol Antimicrobial susceptibility testing against Multidrug resistant gram-negative Bacilli: a comparison of Disk Diffusion to Broth Microdilution. J Clin Microbiol 59(1):1–12. https://doi.org/10.1128/JCM.01649-20
Hackel MA, Tsuji M, Yamano Y et al (2018) In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, Against Carbapenem Nonsusceptible and Multidrug Resistant isolates of Gram-negative Bacilli Collected Worldwide in 2014 to 2016. Antimicrob Agents Chemother 62(2):1–13. https://doi.org/10.1128/AAC.01968-17
Ballesté-Delpierre C, Ramírez Á, Muñoz L et al (2022) Assessment of in Vitro Cefiderocol susceptibility and comparators against an epidemiologically diverse Collection of Acinetobacter baumannii Clinical isolates. Antibiotics 11(2):1–13. https://doi.org/10.3390/antibiotics11020187
Falcone M, Tiseo G, Leonildi A et al (2022) Cefiderocol -compared to colistin- based regimens for the treatment of severe infections caused by Carbapenem Resistant Acinetobacter baumannii. Antimicrob Agents Chemother 66(5):1–12. https://doi.org/10.1128/aac.00065-22
Karakonstantis S, Rousaki M, Kritsotakis E (2022) Cefiderocol: systematic review of mechanisms of Resistance, Heteroresistance and in vivo emergence of resistance. Antibiotics 11(6):1–20. https://doi.org/10.3390/antibiotics11060723
Yao J, Wang J, Chen M et al (2021) Cefiderocol: an overview of its in vitro and in vivo activity and underlying resistant mechanisms. Front Med 8:1–7. https://doi.org/10.3389/fmed.2021.741940
Kohira N, Hackel M, Ishioka Y et al (2020) Reduced susceptibility mechanism to Cefiderocol, a Siderophore Cephalosporin, among clinical isolates from A Global Surveillance Programme (SIDERO-WT-2014). J Glob Antimicrob Resist 22:738–741. https://doi.org/10.1016/j.jgar.2020.07.009
Duman Y, Tekerekoğlu MS (2021) Colistin MICs and Resistance genes of Acinetobacter baumannii isolated in Intensive Care Units. Turk J Intensive Care 19(2):71–75. https://doi.org/10.4274/tybd.galenos.2020.47965
Acknowledgements
None.
Funding
This study was approved by Baskent University Institutional Review Board (Project no: KA22/392) and supported by Baskent University Research Fund.
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Concept, material preparation, data collection and analysis were performed by Aylin USKUDAR-GUCLU, Salih DANYILDIZ, Hasan Cenk MİRZA. Statistical analysis was performed by Mehtap Akcil OK. The first draft of the manuscript was written by Aylin USKUDAR-GUCLU and Salih DANYILDIZ. Hasan Cenk MIRZA and Ahmet BASUSTAOGLU commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declared that there is no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uskudar-Guclu, A., Danyildiz, S., Mirza, H.C. et al. In vitro activity of cefiderocol against carbapenem-resistant Acinetobacter baumannii carrying various β-lactamase encoding genes. Eur J Clin Microbiol Infect Dis 43, 1171–1179 (2024). https://doi.org/10.1007/s10096-024-04831-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-024-04831-w