Abstract
In 2015, an outbreak caused by OXA-48-producing Enterobacteriaceae affected a neonatal intensive care unit at a Swedish University Hospital. The aim was to explore the transmission of OXA-48-producing strains between infants and the transfer of resistance plasmids between strains during the outbreak. Twenty-four outbreak isolates from ten suspected cases were whole-genome sequenced. A complete assembly was created for the index isolate (Enterobacter cloacae) and used as a mapping reference to detect its plasmids in the remaining isolates (17 Klebsiella pneumoniae, 4 Klebsiella aerogenes, and 2 Escherichia coli). Strain typing was performed using core genome MLST and SNP analysis. As judged from sequencing and clinical epidemiological data, the outbreak involved nine cases (two developed sepsis) and four OXA-48-producing strains: E. cloacae ST1584 (index case), K. pneumoniae ST25 (eight cases), K. aerogenes ST93 (two cases), and E. coli ST453 (2 cases). Two plasmids from the index strain, pEclA2 and pEclA4, carrying blaOXA48 and blaCMY-4, respectively, were traced to all K. pneumoniae ST25 isolates. Klebsiella aerogenes ST93 and E. coli ST453 harboured either only pEclA2, or both pEclA2 and pEclA4. One suspected case harbouring OXA-162-producing K. pneumoniae ST37 could be excluded from the outbreak. Once initiated by an E. cloacae strain, the outbreak was caused by the dissemination of a K. pneumoniae ST25 strain and involved inter-species horizontal transfer of two resistance plasmids, one of which carried blaOXA-48. To our knowledge, this is the first description of an outbreak of OXA-48-producing Enterobacteriaceae in a neonatal setting in northern Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last decades, there has been a worrying rise in Gram-negative intestinal bacteria producing carbapenemases, i.e. b-lactamases that hydrolyse carbapenems and usually also other b-lactam antibiotics. These bacteria are particularly troubling since they are often resistant to several additional classes of antibiotics which further limits available treatment options. The incidence of carbapenem-producing Enterobacteriaceae is low but increasing in Sweden: from 0.2 to 1.3 cases/100,000 inhabitants between 2012 and 2021 [1]. Some of the most frequently encountered carbapenemases are those of the OXA-48 group [2]. These enzymes hydrolyse carbapenems only at a low level and show weak activity against cephalosporins, but high-level resistance to carbapenems can be achieved when combined with permeability defects and/or co-expression of extended-spectrum b-lactamases (ESBLs) or AmpC b-lactamases [3, 4]. Carbapenemases of the OXA-48 group, hence, constitute a threat to our ability to treat bacterial infections and it is, therefore, important to understand how blaOXA-48-like genes are disseminated in bacterial populations.
Neonatal care units (NICUs) frequently experience spread of Enterobacteriaceae [5] and outbreaks involving OXA-48-producing Enterobacteriaceae strains in this setting have been reported [6,7,8]. Due to sparse colonization by anaerobic bacteria during the first weeks of life, members of the Enterobacteriaceae family establish and reach high abundance levels in the neonatal gut. As the gut microbiota matures, the proliferation of Enterobacteriaceae and other facultative anaerobic bacteria is suppressed [9]. The acquisition of anaerobes is severely delayed in preterm infants, partly related to comprehensive antibiotic treatments, rendering this population prone to heavy colonization by Enterobacteriaceae [10]. A high concentration of these bacteria in the gut lumen may favour bacterial conjugation and plasmid transfer, which requires close contact between bacterial cells[11,12,13]. The intestines of preterm infants could therefore represent a hotspot for horizontal transfer of blaOXA-48 and other plasmid-borne resistance genes, as also indicated by a recent experimental study [14].
In this study, we describe an outbreak in a NICU at a Swedish University Hospital. The outbreak involved ten suspected cases and four different OXA-48-producing Enterobacteriaceae species: Enterobacter cloacae, Klebsiella pneumoniae, Klebsiella aerogenes, and Escherichia coli. We used whole-genome sequencing to identify both clonal spread between cases and the dissemination of plasmids, originating in the index strain (E. cloacae), to other strains. The results strongly indicate horizontal transfer in vivo—between both species and genera—of two different plasmids encoding the carbapenemase OXA-48 and the AmpC-type b-lactamase CMY-4, respectively.
Materials and methods
The setting, data collection, and definitions
The outbreak occurred in 2015 in an NICU of a tertiary care University Hospital in Western Sweden. Comprised of 14 beds allocated to six rooms, the ward provides specialist care for approximately 1000 term and preterm infants (born from gestational week 22 + 0) annually. In 2015, rectal screening cultures were obtained from all patients at admission to the ward and weekly thereafter. If carriage of ESBL- or carbapenemase-producing bacteria was suspected or confirmed by the Clinical Microbiology laboratory, the patient was cared for in a single-bed room by a dedicated nurse.
Details on contact tracing and sampling were retrospectively collected from the patient charts and reports from the infection prevention and control team. A suspected case was defined as a patient within the NICU from whom an Enterobacteriaceae strain positive for carbapenemases of the OXA-48 group was isolated. The outbreak period was defined from the identification of the first suspected case—i.e. the sampling date of the case’s first positive isolate—to the identification of the last suspected case. No additional infants colonized or infected by Enterobacteriaceae positive for carbapenemases of the OXA-48-group were identified at the NICU past this period within the following year. (Fig. 1, Supplementary Fig. S1).
Timeline of the outbreak with OXA-48-producing Enterobacteriaceae in a neonatal unit. Isolation day, species identity and carriage of the outbreak plasmids are shown for the isolates selected from the ten suspected cases, with the index isolate (Case 1) set as day 0. Twin pair one (*) and twin pair two (**) are marked with asterisks. Horizontal lines represent the duration of hospital care, and their colour indicates the ward caring for each case until case identification: red = Ward A, blue = Ward B, black = Ward C, orange = Ward D. Symbols represent species: star = E. cloacae, square = K. pneumoniae, triangle = K. aerogenes, and circle = E. coli. Carriage of outbreak plasmids is indicated by symbol colour: green = carriage of pEclA2, yellow = carriage of pEclA4, white = no carriage of outbreak plasmids
The study was approved by the Regional Ethical Review Board in Gothenburg, Sweden (no. 997–18 and no. 228–15).
Culturing, bacterial identification and characterization, and selection of isolates
During the outbreak period, ten infants were colonized and/or infected by Enterobacteriaceae positive for OXA-48-like carbapenemases. The methods used for culturing, bacterial identification, antimicrobial susceptibility testing, and identification of resistance determinants were those routinely applied in the clinical microbiology laboratory at the University Hospital (Supplementary Material). A total of 155 rectal screening samples were obtained from the ten suspected outbreak cases during and after the outbreak period, of which 78 were assessed as positive for Enterobacteriaceae bacteria producing carbapenemases of the OXA-48 group (Supplementary Figure S1). In addition, four clinical samples—from two of the suspected cases (two blood cultures, two nasopharyngeal cultures) —were positive for such bacteria (Supplementary Figure S1). In total, 116 isolates assessed as positive for carbapenemases of the OXA-48 group were obtained from the 82 positive samples and 74/116 isolates were saved and stored frozen (− 80 °C) at the Clinical Microbiology laboratory.
For this study, we selected 24 of the stored isolates with confirmed carriage of genes encoding a carbapenemase of the OXA-48 group. For each suspected case, the first identified isolate of each OXA-48-positive species colonizing that infant was selected (n = 14). For Case 2, the first identified isolate was a blood culture isolate, and both the blood isolate and the rectal screening isolate from the following day were selected for analysis. Furthermore, from three persistently colonized cases, nine isolates were selected that showed deviations in phenotypic resistance tests and/or meropenem MIC-values from previous isolates of that species in the colonized infant. In total, 1 E. cloacae, 17 K. pneumoniae, 4 K. aerogenes, and 2 E. coli isolates were selected for whole-genome sequencing. (Table 1, Supplementary Figure S1).
DNA extraction and whole-genome sequencing
Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen) for short-read sequencing and the Marmur protocol for long-read sequencing [15]. DNA quality and quantity were measured with a Nanodrop™ ND-1000 Spectrophotometer (Thermo Fisher) and a Qubit 2.0 Fluorometer (Invitrogen), respectively. The species identity was confirmed by 16S rRNA Sanger sequencing (GATC Biotech). The isolates were whole genome sequenced using either IonTorrent (isolates Ecl1A, Kpn2A2, Kpn5B, Kpn6A, Kpn7C, Kpn8A1, Kpn9A2, Kpn10A1) or Illumina (isolates Kpn2A1, Kpn3A1, Kae3B2, Kpn3B3, Kpn3B4, Kae3D5, Kae3D6, Kpn4A, Eco8A2, Kpn8A3, Kpn8E4, Kae9A1, Kpn10A2, Kpn10A3, Eco10A4, Kpn10E5) technology. The index isolate (Ecl1A) was also sequenced by Nanopore (full details in Supplementary material). All sequenced isolates were deposited at the Culture Collection University of Gothenburg (CCUG; Table 1).
Sequence data analysis
The Illumina sequence data were trimmed from adapters and low-quality sequences using Trim galore! (www.bioinformatics.babraham.ac.uk/projects/trim_galore/; v. 0.4.3, parameters -stringency 3 -paired -retain_unpaired). The short-read data for all but the index isolate was assembled using SPAdes (v.3.14.1, Illumina parameters: -isolate -cov-cutoff 5; IonTorrent parameter: -iontorrent -k 21,33,55,77,99,127 -isolate -cov-cutoff 5) [16]. The short- and long-read data for the index isolate were assembled using Unicycler (v. 0.4.4, default parameters) [17] which produced complete circular assemblies of the chromosome and all plasmids. This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JAGPNS000000000-JAGPOP000000000. The versions described in this paper are versions JAGPNS010000000.1- JAGPOP010000000.1 (Table 1).
The assemblies were annotated for antimicrobial resistance genes using ResFinder followed by manual inspection (v. 4.1, 90% identity, 20% coverage) [18] and the incompatibility groups of the index isolate plasmids were identified using PlasmidFinder (v. 2.1, default parameters). To investigate the transfer of the index isolate’s plasmids, the reads of the remaining isolates were mapped to the plasmid assemblies in the following way. First, the IonTorrent and the trimmed Illumina data were error-corrected using SPAdes (v 0.4.3, parameters: Illumina, -only-error-correction; IonTorrent, -only-error-correction -iontorrent) [16]. The trimmed and error-corrected paired reads were mapped to each of the five plasmids of the index isolate using BowTie2 (v. 2.3.0, default parameters) [19] and the coverage was calculated for each position using samtools (v 1.6) [20]. The complete plasmid profiles of isolates other than the index isolate were not investigated.
Epidemiological typing of all sequenced isolates was performed using MLST schemes from PubMLST.org [21]. All K. pneumoniae sequences were also submitted to the Institute Pasteur MLST-2.0 server and analysed with the BIGSdb database for curation and publication of core genome MLST [22]. Epidemiological strain typing of E. coli and K. aerogenes isolates was done by SNP analysis using the references E. coli IAI1 (accession number ENA_CU928160 and K. aerogenes KCTC 2190 (NC_015663.1) in CLC Genomics Workbench 12 (Qiagen; full details in Supplementary materials).
Results
Outbreak description
The outbreak timeline and the follow-up period are shown in Fig. 1 and Supplementary Figure S1. During the outbreak, Enterobacteriaceae strains producing carbapenemases of the OXA-48 group were isolated from two twin pairs and six singlets, with a mean gestational age of 27 weeks (clinical characteristics of the 10 preterm infants are shown in Supplementary Table S1). All suspected cases except the index case (Case 1) yielded a K. pneumoniae isolate positive for OXA-48-like carbapenemases at the time of case identification. Case 2 was identified from a positive blood culture and the others were identified by rectal screening.
The index case was identified at Ward A, where an E. cloacae isolate positive for the OXA-48 group (Ecl1A) was detected in a rectal screening culture collected on day 0 (Table 1, Fig. 1, Supplementary Fig. S1). Due to overcrowding, the patient was initially cared for in a multi-bedroom by a dedicated nurse before being transferred to a single-bed room on day 5. Since isolation routines had not been followed, rectal screening was enhanced to twice weekly from day 5, including all neonates at the ward. The index case remained on Ward A for 10 days, but no follow-up cultures were obtained. The second case was identified on day 17 on Ward A, by a blood culture yielding a K. pneumoniae isolate positive for the OXA-48 group (Kpn2A1). Rectal screening the following day detected gut colonization by similar OXA-48-producing K. pneumoniae in Cases 2–5. At this time point, Case 5 had been transferred to a different unit (Ward B), where Case 6 was discovered 4 days later (day 22). On day 29, Case 7 was identified at a third unit (Ward C). Three additional cases (8–10) were identified on Ward A between days 35 and 49: Cases 8 and 10 were a twin pair and Case 9 was the twin sibling of Case 3. Thus, all suspected cases except for Case 7 had a spatial link to either Ward A or Ward B and/or another case.
Screening cultures from the two twin pairs also yielded gut colonization with other enterobacterial species positive for OXA-48-like carbapenemases. Klebsiella aerogenes was isolated from Case 3 (Kae3B2) on day 28 and 11 days later from the sibling, Case 9, who, thus, harboured both K. aerogenes (Kae9A1) and K. pneumoniae (Kpn9A2) strains positive for the OXA-48 group at the time of case identification. A positive E. coli strain was first isolated from Case 8 (Eco8A2) on day 74, i.e. after the outbreak period, and 14 days later from its sibling, Case 10 (Eco10A4).
The infection prevention and control team identified overcrowding, deficient routine practices for milk preparation, inadequate handling of shared medical equipment, inexperienced healthcare workers, and cluttering of non-medical utensils in the patient areas as risk factors for continued transmission. Hence, control measures were focused on adherence to existing routines for infection prevention and control and included the education of both healthcare workers and parents. The extended rectal screening program was continued until day 60.
Two infants, Case 2 and Case 8, developed sepsis at 28 and 39 days of age, respectively, with blood cultures yielding K. pneumoniae positive for OXA-48-like carbapenemases. Of the six cases that were screened more than one year after the outbreak, three were still colonized by OXA-48-producing Enterobacteriaceae: two with K. pneumoniae (Cases 8 and 10) and one with K. aerogenes (Case 3). Later follow-up samples from these infants were negative (Supplementary Figure S1).
The index isolate and its plasmids
The index isolate E. cloacae CCUG 67,822 (Ecl1A) belonged to a novel sequence type (ST), ST1584 [23]. All its plasmids, except for the smallest one (pEclA5), harboured antimicrobial resistance genes (Table 2). The blaOXA-48 gene was located as the only resistance gene on a 64 kb long IncL plasmid (pEclA2), which was near identical (two substitutions, both non-synonymous, in traN and traX) to the wide-spread OXA-48-encoding plasmid characterized by Hamprecht et al. [24]. This plasmid locates blaOXA-48 between two identical insertion sequences, IS1999, members of the transposon (Tn)1999 family [2]. The plasmid pEclA4 also carried a single resistance gene, blaCMY-4, and was almost identical (one substitution and one insertion, both in an intergenic region) to the CMY-4-encoding IncQ plasmid described by Kotsakis et al. [25]. The largest plasmid, pEclA1, carried genes encoding resistance towards β-lactams (blaTEM-1), sulphonamides (sul2), aminoglycosides (aac(3)-IIa), and fluoroquinolones (qnrS1). Finally, the plasmid pEclA3 harboured resistance genes towards β-lactams (blaTEM-1), sulphonamides (sul1) aminoglycosides (aadA1), trimethoprim (dfrA15), tetracyclines (tet(D)), and chloramphenicol (catA2; Table 2).
Detection of plasmids pEclA2 and pEclA4 in other outbreak isolates
By mapping the short reads of the 23 sequenced non-index isolates onto the complete plasmid sequences of the index isolate, we could conclude that all isolates harboured a plasmid resembling pEclA2, i.e. the blaOXA-48 plasmid (Fig. 1, Tables 2 and 3). All isolates had a uniform mapping coverage over pEclA2, except for the K. pneumoniae isolate from Case 7 (Kpn7C), in which the plasmid lacked a 12 kb region (position 31 267–43 887) including, e.g. genes involved in conjugation (mobAB and traHIJ), as well as a 1.5-kb region (position 46 682–46 792) encoding a primase. Further, the plasmid of isolate Kpn7C did not harbour blaOXA-48 but blaOXA-162, another carbapenemase of the OXA-48 group. This isolate also lacked pEclA4, the IncQ plasmid of the index isolate, which was detected in all other K. pneumoniae isolates, one K. aerogenes, and one E. coli isolate (Fig. 1, Table 3, Fig. 2). None of the other plasmids of the index isolate was found in the other outbreak isolates.
Epidemiological strain typing
The MLST analysis revealed that all K. pneumoniae isolates belonged to ST25 except for isolate Kpn7C (Case 7) which belonged to ST37 (Table 3). Further analyses using core genome MLST (573 loci) showed no more than two allele differences between the ST25 isolates, strongly indicating strain identity. Isolate Kpn7C clearly belonged to a separate strain as it differed in at least 475 core alleles when compared to the ST25 isolates. Based on these data, and the fact that the blaOXA-162 plasmid of isolate Kpn7C did not fully resemble pEclA2, we could conclude that Case 7 was not part of the outbreak.
The four K. aerogenes isolates (Kae3B2, Kae3D5, Kae3D6, and Kae9A1) from twin pair 1, Case 3 and 9, belonged to ST93 (Table 3), and the SNP analysis of these isolates identified 1–7 SNPs difference using K. aerogenes KCTC 2190 as the reference and 0–4 SNPs using Kae3D5 as the reference, demonstrating that the four isolates belonged to the same strain. Finally, the two E. coli isolates (Eco8A2 and Eco10A4) from twin pair 2, Cases 8 and 10, both belonged to ST453 (Table 3), and the SNP analysis did not reveal any true SNPs when comparing the two isolates.
Discussion
In this study, we describe in detail an outbreak caused by OXA-48-producing Enterobacteriaceae, involving nine preterm infants at a Swedish NICU. To the best of our knowledge, this is the first description of an outbreak of OXA-48-producing Enterobacteriaceae in a neonatal setting in northern Europe.
Interestingly, all cases identified in the present outbreak were preterm infants (gestational age at birth 24–34 weeks). Both prematurity per se, but also increased exposure to antibiotics in this group may hamper colonization by protective anaerobic gut bacteria [10]. This predisposes to overgrowth by Enterobacteriaceae, and high population counts of these bacteria might promote bacterial conjugation and horizontal gene transfer [14, 26].
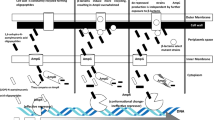
The extended rectal screening program, initiated after the identification of the index case, ensured early detection of each new case and in-depth molecular data was obtained using whole-genome sequencing. The approach of combining genomic and clinical epidemiological data allowed us to create a likely scenario for the plasmid transfer and strain dissemination (Fig. 2).
Thus, we propose that the E. cloacae index strain transferred two plasmids, pEclA2 and pEclA4, to the outbreak strain K. pneumoniae ST25. This likely occurred in the gut of Case 1, who was identified as both the index and the primary case in this outbreak. However, since Case 1 was excluded from further screening after the isolation of the E. cloacae strain, carriage of K. pneumoniae ST25 could not be established for this patient. Therefore, an unidentified carrier in whom the plasmid transfer occurred, either from E. cloacae ST1584 to K. pneumoniae ST25 or the reverse, cannot be excluded, although the meticulous rectal screening program reduces the likelihood of an undetected neonatal case within Ward A. It is also unlikely that the plasmid transfer occurred outside a human host since high bacterial densities are required for conjugation [26].
This outbreak lasted for 50 days; hence, multiple incidents would have contributed to the continued spread of the K. pneumoniae ST25 strain. However, the simultaneous identification of Cases 2–5, after several negative screening cultures, indicates that they obtained the K. pneumoniae ST25 strain at approximately the same time, although the different resistance profiles of their isolates could imply several sources of transmission (Table 2). As Case 1 had been absent from the ward for 7 days at the time of their identification, we hypothesize that K. pneumoniae ST25 spread from Case 1 to Cases 2–5 through one or several contaminated fomites within Ward A. This could not be further investigated as no environmental cultures were collected during the outbreak. However, the isolation of outbreak clones of K. pneumoniae from the NICU environment has been reported in several studies [7, 27,28,29,30,31].
Within Ward B, K. pneumoniae ST25 was likely transmitted from Cases 5 to Case 6 since there was a clear spatial and temporal link between these cases. The source and route of spread to Case 8 were obscure, while the discovery of Cases 9 and 10 was expected due to the spread from their respective twin sibling.
The genetic analysis of isolate Kpn7C separated Case 7 from the outbreak. This illustrates the potential clinical benefits of access to rapid epidemiological genetic typing in future outbreak investigations, as unnecessary resource-consuming investigations may be avoided at an early stage of an outbreak.
Although we could only speculate on when and where the plasmids pEclA2 and pEclA4 have transferred between the index E. cloacae strain and the K. pneumoniae outbreak strain, other plasmid transfer events were more clearly indicated. Based on the genetic analysis and timeline of follow-up isolates from the two twin pairs, in vivo horizontal transfer of the plasmids pEclA2 and pEclA4 from the outbreak K. pneumoniae strain to other enterobacterial species was strongly suggested (Fig. 1). We hypothesize that an initial transfer of pEclA2, followed by pEclA4 from K. pneumoniae ST25 to the K. aerogenes ST93 strain occurred in the gut of Case 3, and that both the K. pneumoniae ST25 strain (with both plasmids) and the K. aerogenes ST93 strain (with plasmid pEclA2) were transmitted to its sibling, Case 9. Similarly, we propose that pEclA2 and pEclA4 were transferred from the K. pneumoniae ST25 strain to the E. coli ST453 strain in the gut of Case 8. Somewhat later the E. coli ST453 strain carrying only the pEclA2 plasmid was isolated from its twin sibling, Case 10. This could either be a result of the transfer of pEclA2 from K. pneumoniae ST25 to E. coli ST453 in the gut of Case 10, or the spread of an E. coli ST453 variant carrying only pEclA2 from Cases 8 to Case 10.
Our data, therefore, give strong indications, but no proof of plasmid transfer. However, transfer of resistance plasmids in vivo between E. coli strains in the gut microbiota of infants has previously been demonstrated [13, 32], and in vitro conjugation experiments have shown horizontal transfer of carbapenemase-encoding outbreak plasmids between gut bacteria [24, 33, 34]. Further, a recent experimental study suggests a high potential for horizontal gene transfer in the gut microbiota of preterm neonates [14].
In summary, strain typing and plasmid tracing using whole-genome sequencing in combination with clinical epidemiology data proved to be a useful tool for the analysis of the transmission of bacterial strains and in vivo transfer of plasmids during this outbreak. Our methods allowed the exclusion of a strain producing an OXA-48-like carbapenemase unrelated to the outbreak, while several enterobacterial strains of different species could be identified as part of the outbreak, due to their carriage of identical OXA-48-encoding plasmids. Although rapid less discriminating diagnostic methods are crucial for an early outbreak response, the ability to discriminate non-related cases at an early stage is important for effective resource allocation. Even more so in high-prevalence settings where incidental findings of carbapenemase-producing Enterobacteriaceae are common.
Data Availability
The genome assemblies of the included isolates have been submitted til NCBI Assembly. The accession numbers for the assemblies are listed in Table 1 together with additional information on the included isolates.
References
Institute PHAoSaNV, Swedres Svarm- sales of antibiotics and occurrence of antibiotic resistance in Sweden, in Swedres Svarm, R.O.a.W.T. Olov Aspevall, Public health agency of Sweden. Oskar Nilsson and Märit Pringle, National Veterinary Institute., Editor. 2021, Public Health Agency of Sweden and National Veterinary Institute: Sweden
Pitout JDD, Peirano G, Kock MM, Strydom KA, Matsumura Y (2019) The global ascendency of OXA-48-Type Carbapenemases. Clin Microbiol Rev 33(1):e00102-19. https://doi.org/10.1128/CMR.00102-19
Poirel L, Potron A, Nordmann P (2012) OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67(7):1597–1606
Mansour W et al (2017) Outbreak of colistin-resistant carbapenemase-producing Klebsiella pneumoniae in Tunisia. J Glob Antimicrob Resist 10:88–94
Flannery DD et al (2022) Neonatal multidrug-resistant gram-negative infection: epidemiology, mechanisms of resistance, and management. Pediatr Res 91(2):380–391
Taoufik L et al (2019) Emergence of OXA-48 carbapenemase producing Klebsiella pneumoniae in a neonatal intensive care unit in Marrakech. Morocco Clin Med Insights Pediatr 13:1179556519834524
Adler A et al (2013) Epidemiological and microbiological characteristics of an outbreak caused by OXA-48-producing Enterobacteriaceae in a neonatal intensive care unit in Jerusalem. Israel J Clin Microbiol 51(9):2926–2930
Gona F et al (2019) Emergence of two novel sequence types (3366 and 3367) NDM-1- and OXA-48-co-producing K pneumoniae in Italy. Eur J Clin Microbiol Infect Dis 38(9):1687–1691
Beller L, Deboutte W, Falony G, Vieira-Silva S, Tito RY, Valles-Colomer M, Rymenans L, Jansen D, Van Espen L, Papadaki MI, Shi C, Yinda CK, Zeller M, Faust K, Van Ranst M, Raes J, Matthijnssens J (2021) Successional Stages in Infant Gut Microbiota Maturation. mBio 12(6):e0185721. https://doi.org/10.1128/mBio.01857-21
Aguilar-Lopez M et al (2021) A systematic review of the factors influencing microbial colonization of the preterm infant gut. Gut Microbes 13(1):1–33
Lerminiaux NA, Cameron ADS (2019) Horizontal transfer of antibiotic resistance genes in clinical environments. Can J Microbiol 65(1):34–44
Neil K, Allard N, Rodrigue S (2021) Molecular mechanisms influencing bacterial conjugation in the intestinal microbiota. Front Microbiol 12:673260
Gumpert H et al (2017) Transfer and persistence of a multi-drug resistance plasmid in situ of the infant gut microbiota in the absence of antibiotic treatment. Front Microbiol 8:1852
Hagbo M et al (2020) Experimental support for multidrug resistance transfer potential in the preterm infant gut microbiota. Pediatr Res 88(1):57–65
Francisco Salvà-Serra, Margarita Gomila, Liselott Svensson-Stadler et al (2018) A protocol for extraction and purification of high-quality and quantity bacterial DNA applicable for genome sequencing: a modified version of the Marmur procedure. PROTOCOL (Version 1) available at Protocol Exchange. https://doi.org/10.1038/protex.2018.084
Bankevich A et al (2012) SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19(5):455–477
Wick RR et al (2017) Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13(6):e1005595
Zankari E et al (2012) Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67(11):2640–2644
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9(4):357
Li H et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25(16):2078–2079
Larsen MV et al (2012) Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50(4):1355–1361
Jolley KA, Bray JE, Maiden MCJ (2018) Open-access bacterial population genomics: BIGSdb software, the PubMLST org website and their applications. Wellcome Open Res 3:124
Miyoshi-Akiyama T et al (2013) Multilocus sequence typing (MLST) for characterization of Enterobacter cloacae. PLoS ONE 8(6):e66358
Hamprecht A et al (2019) Pathogenicity of Clinical OXA-48 Isolates and impact of the OXA-48 IncL plasmid on virulence and bacterial fitness. Front Microbiol 10:2509
Kotsakis SD et al (2015) Characterization of a mobilizable IncQ plasmid encoding cephalosporinase CMY-4 in Escherichia coli. Antimicrob Agents Chemother 59(5):2964–2966
Stecher B et al (2012) Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc Natl Acad Sci USA 109(4):1269–1274
Haller S et al (2015) What caused the outbreak of ESBL-producing Klebsiella pneumoniae in a neonatal intensive care unit, Germany 2009 to 2012? Reconstructing transmission with epidemiological analysis and whole-genome sequencing. BMJ Open 5(5):e007397
Rettedal S et al (2012) First outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a Norwegian neonatal intensive care unit; associated with contaminated breast milk and resolved by strict cohorting. APMIS 120(8):612–621
Giuffre M et al (2013) Successful control of an outbreak of colonization by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae sequence type 258 in a neonatal intensive care unit. Italy. J Hosp Infect. 85(3):233–6
Saporito L et al (2021) Efficacy of a coordinated strategy for containment of multidrug-resistant Gram-negative bacteria carriage in a Neonatal Intensive Care Unit in the context of an active surveillance program. Antimicrob Resist Infect Control 10(1):30
Cantey JB, Sreeramoju P, Jaleel M, Treviño S, Gander R, Hynan LS, Hill J, Brown C, Chung W, Siegel JD, Sánchez PJ (2013) Prompt control of an outbreak caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit. J Pediatr 163(3):672–9.e1–3. https://doi.org/10.1016/j.jpeds.2013.03.001
Porse A et al (2017) Genome Dynamics of Escherichia coli during antibiotic treatment: transfer, loss, and persistence of genetic elements in situ of the infant gut. Front Cell Infect Microbiol 7:126
Tofteland S et al (2013) A long-term low-frequency hospital outbreak of KPC-producing Klebsiella pneumoniae involving Intergenus plasmid diffusion and a persisting environmental reservoir. PLoS ONE 8(3):e59015
Pitart C et al (2011) First outbreak of a plasmid-mediated carbapenem-hydrolyzing OXA-48 beta-lactamase in Klebsiella pneumoniae in Spain. Antimicrob Agents Chemother 55(9):4398–4401
Acknowledgements
The authors acknowledge the assistance of the Culture Collection University of Gothenburg (CCUG) Lab. The CCUG is supported by the Department of Clinical Microbiology at the Sahlgrenska University Hospital. We thank the Institute Pasteur teams for the curation and maintenance of BIGSdb-Pasteur databases at bigsdb.pasteur.fr.
Funding
Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hallbäck, E.T., Johnning, A., Myhrman, S. et al. Outbreak of OXA-48-producing Enterobacteriaceae in a neonatal intensive care unit in Western Sweden. Eur J Clin Microbiol Infect Dis 42, 597–605 (2023). https://doi.org/10.1007/s10096-023-04584-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04584-y