Abstract
We investigated the faecal carriage prevalence of extended-spectrum β-lactamase production in Escherichia coli (EP-EC) and/or Klebsiella pneumoniae (EP-KP) and risk factors associated with carriage among adult study subjects in Finland, Germany, Latvia, Poland, Russia and Sweden (partner countries). The aim was to get indicative data on the prevalence of ESBL-carriage in specific populations in the region. Faecal samples were collected from four study populations and screened on ChromID-ESBL and ChromID-OXA-48 plates. Positive isolates were further characterised phenotypically. Our results show a large variation in carrier prevalence ranging from 1.6% in Latvia to 23.2% in Russia for EP-EC. For the other partner countries, the prevalence of EP-EC were in increasing numbers, 2.3% for Germany, 4.7% for Finland, 6.6% for Sweden, 8.0% for Poland and 8.1% for all partner countries in total. Carriers of EP-KP were identified only in Finland, Russia and Sweden, and the prevalence was < 2% in each of these countries. No carriers of carbapenemase-producing isolates were identified. This is the first study reporting prevalence of carriers (excluding traveller studies) for Finland, Latvia, Poland and Russia. It contributes with important information regarding the prevalence of EP-EC and EP-KP carriage in regions where studies on carriers are limited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asymptomatic faecal carriage of extended-spectrum β-lactamase-producing bacteria like Escherichia coli (EP-EC) or Klebsiella pneumoniae (EP-KP) contributes to dissemination of antibiotic resistance in the community which could ultimately lead to increased resistance prevalence in community-acquired infections. One important risk factor for acquiring a bloodstream infection (BSI) with ESBL-producing Enterobacteriaceae is a previous ESBL-positive culture (carriage or infection) [1,2,3]. The number of people at risk for ESBL-BSI could therefore increase if community carriage of EP-EC and EP-KP increases. Potential roles of carriers in the epidemiology of EP-EC and EP-KP are however not clear. Carriers in the community could serve as a link between resistance in animals and environment and clinical infections in humans [4,5,6,7]. However, the role of carriers has not been addressed in large surveillance initiatives. Extensive knowledge of the antibiotic resistance epidemiology is important for rational and effective antibiotic use for both hospital and community-acquired infections [8].
Most studies of ESBL-carriers have addressed the risk of becoming a carrier when travelling to high prevalence countries, a risk that has been established in several studies [9,10,11,12]. However, the prevalence and risk factors associated with carriage in different populations in the community is not well studied.
Recruiting healthy study subjects is challenging since they in general are hard to reach and their participation is either relying on their will to do good for public health without personal gain or monetary compensation. Populations like university students, army recruits and primary care visitors are often recruited to be investigated for carrier prevalence [13]. Germany and Sweden have performed separate large studies on carriers, with study subjects representing a cross section of the population, where the carrier prevalence of EP-EC reported were at 6.3% and 4.7%, respectively [14, 15].
We designed a cross-sectional study on the prevalence of intestinal EP-EC and EP-KP carriers in study populations in Finland, Germany, Latvia, Poland, Russia and Sweden (referred to as partner countries) simultaneously in 2015–2017. The aim was to investigate the prevalence in countries where carriage of EP-EC and EP-KP had never been studied and to follow up on the development in countries that had previous studies on carriage. Moreover, we wanted to build capacity in the respective countries to perform studies on asymptomatic carriage. Information on possible risk factors for EP-EC and EP-KP carriage were collected by a questionnaire directed to all study subjects.
Materials and methods
Common study protocols for collection of faecal samples and questionnaires were developed and approved by all partners. Written informed consent was obtained from all study subjects included in the study. Ethical permission was gained by each partner country individually.
Each country sampled from the following regions: Turku and Helsinki region (Finland), Riga region (Latvia), Leipzig region (Germany), Silesian voivodeship (Poland), Smolensk region (Russia), Stockholm region (Sweden). Samples were collected between October 2015 and January 2017 with the aim to collect 250 samples per country. Faecal samples were collected by 1 ml Eswab (Copan Diagnostics Inc., Mantua, Italy) from populations defined beforehand by partners. Defined sample populations were individuals visiting different types of primary care centres (defined as non-inpatient care), elective day surgery patients, university students and advertising on internet-based sites for recruiting study subjects. Women and men between 18 and 65 years that had resided in the country for at least 1 year, not taken antibiotics during the last 3 months and that did not have current diarrhoea at the time of sampling were included in the study. In Germany, only the age criteria was enforced and not the other exclusion criteria.
Faecal samples were cultured by plating 100 μl of liquid from the Eswab Collection Kit medium on each of the commercially available plates, ChromeID ESBL and ChromeID OXA-48 (bioMérieux, Marcy-l’Étoile, France) and incubated for 18–24 h in 35–37 °C. After the incubation, colonies were isolated and species identification determined by MALDI-TOF (Biotyper, Bruker Corporation, the Netherlands). Antibiotic susceptibility tests were performed on E. coli and K. pneumoniae isolates with disc diffusion and gradient tests according to EUCAST methodology and breakpoints (http://www.eucast.org/clinical_breakpoints/ accessed on 2018-07-06). Resistance rates were defined as the percentage of non-susceptible (R + I) isolates to the antibiotic in question. For Germany, equivalent methods for selection of resistant isolates with CHROMagar™ ESBL and CHROMagar™ KPC plates were used and the methods were previously published [11].
Phenotypic β-lactamase identification was performed with combination disc testing with clavulanic acid in combination with cefotaxime or ceftazidime for ESBL-production (Becton Dickinson, Cockeysville, MD, USA) and cloxacillin in combination with cefotaxime or ceftazidime for AmpC-production (Rosco Diagnostica A/S, Taastrup, Denmark). For carbapenemase-production, the following neosensitab combinations were used: meropenem with phenylboronic acid for detecting KPC, meropenem with dipicolinic acid for detecting MBL and temocillin for detecting OXA-48 (KPC, MBL and OXA-48 Confirmation Kit, Rosco Diagnostica A/S, Taastrup, Denmark). Multi-drug resistance (MDR) was defined according to Magiorakos et al. 2012 as resistance to three or more classes of antibiotics tested [16].
All study subjects received the same questionnaire (translated to respective native language) in all partner countries (Online resource 1).
All data from partner countries were reported, summarised and analysed using the same methods and performed at the Public Health Agency of Sweden. Univariate risk analysis was performed only for non-carriers and EP-EC-carriers that had both contributed with a sample and a questionnaire. Carriers and non-carriers were also compared between countries within different subpopulations (between-country analyses). Firth’s logistic regression model was used to calculate odds ratios and confidence intervals for the risk analyses. All statistical analysis was performed in R statistical software (version 3.2.5).
Results
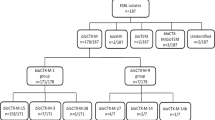
In total, 99 EP-EC and 8 EP-KP were isolated from 1211 faecal samples collected from study populations in Finland, Germany, Latvia, Poland, Russia and Sweden (Tables 1 and 2). Samples were collected from primary care 44.3%, adverts 22.6%, university students 19.5% and elective day surgery 13.5% and the study populations varied between partner countries (Table 1).
The prevalence of EP-EC carriers varied from 1.6% in Latvia to 23.2% in Russia with a total prevalence of 8.1% for all partner countries (Table 1). Prevalence of EP-EC in other partner countries were in increasing numbers, 2.3% for Germany, 4.7% for Finland, 6.6% for Sweden and 8.0% for Poland (Fig. 1). For EP-KP, no carriers were identified in Germany, Latvia and Poland, while Finland, Russia and Sweden identified 2 (1.1%), 5 (2.0%) and 1 (0.3%) carriers, respectively, (Fig. 1). No carriers of carbapenemase-producing isolates including OXA-48-like producers were identified. In Russia, two carriers were identified that were positive for both EP-EC and EP-KP. Neither of these study subjects had been hospitalised during the last 6 months but one had taken antibiotics the last 12 months and worked within the health care sector.
Susceptibility testing (Table 2) of EP-EC showed over 35% resistance to trimethoprim, trimethoprim-sulfamethoxazole and ciprofloxacin. Moreover, 87% of EP-EC were MDR in total for all partner countries (Table 2). Low overall resistance rates (less than 11%) were recorded for nitrofurantoin, mecillinam, meropenem and fosfomycin. For the 8 EP-KP isolates, the antibiotic resistance profiles resembled those of EP-EC (Table 2).
A risk analysis for each partner country was performed to investigate potential risk factors within each country (Online resource 2). Risk factors were only identified for Russian study subjects, the age groups 31–50 and 51–65 years were at higher risk for being carriers compared to study subjects that were 18–30 years (OR 2.3 for both ages Online resource 2). Also Russian study subjects that had been hospitalised during the last 6 months were at higher risk for being carriers compared to no hospitalisation in Russia (OR 5.2) Online resource 2.
Low number of carriers, who travelled to different destinations, did not allow for a valid risk analysis. No risk analysis was performed for EP-KP carriers due to too few positive study subjects. Pooling of EP-EC and EP-KP isolates in the risk analysis was not considered due to general differences in epidemiology of the species [17].
Discussion
In this multi-country study, we investigated the carrier point prevalence of EP-EC and EP-KP and showed large differences between the study populations in Finland, Germany, Latvia, Poland, Russia and Sweden where Russia had considerably higher numbers of EP-EC carriers compared to the other partner countries (Table 1, Fig. 1). This is the first carrier prevalence study (excluding traveller studies) ever reported for Finland, Latvia, Poland and Russia. The geographical setting, including countries from Northern and Eastern Europe and Russia, is also unique since comparisons between these countries are lacking. No carriers of carbapenemase-producing E. coli and K. pneumoniae (including OXA-48-like producers) were identified, which indicate that the prevalence of these is likely to be low. Carriers of EP-KP were identified in half of the partner countries but the prevalence was not exceeding 2%, which suggests a low overall prevalence in the community.
Although parameters such as antibiotic treatment (18–39%), hospitalisation (2–8%) and age (median 28–43 years) differed between countries (Table 1), none of these factors alone explains the higher prevalence seen for Russia since other countries had study populations that were older (Poland), had higher antibiotic treatment (Latvia) or hospitalisation history (Poland).
The large difference in the prevalence of carriers seen between the neighbouring countries Latvia and Russia (1.6 vs 23.2%) could be due to variation in study population characteristics. However, age, sex, antibiotic treatment and hospitalisation history were similar in Russia and Latvia (Table 1) indicating that our results reflect an actual difference in carriage rate between these countries in the studied populations.
The high prevalence reported for Russian carriers was not unexpected since widespread dissemination of ESBL-producers in Russian hospitals has started earlier than in many European countries and has been reported exceeding 60% in recent studies [18,19,20]. Regarding the risk analysis, Russia was the only country where risk factors could be identified (higher age than 30 and previous hospitalisation, Online resources 2) but further studies are needed to establish if these are valid risk factors.
Sweden and Finland showed a similar prevalence, which is also supported by results from other surveillance initiatives [17, 21, 22]. This is likely due to similarities in healthcare structure, antibiotic stewardship and travel patterns in the general population. A Finnish travellers study conducted between 2009 and 2010 showed a prevalence of 1.2% pre-travel, which was lower than what we found in this study. However, a different study population and an earlier study date could explain the higher prevalence seen in our study [10]. In Sweden, a previous study identified a 4.7% national carrier prevalence [15]. The 6.6% identified in this study could be an increase over time but it is hard to draw any conclusion since the method for recruitment of study subjects was different.
The prevalence for German study subjects (2.3%) was lower than expected since previous studies in a similar setting have shown prevalence numbers of 6.3–6.8% [11, 14].
The only previous data on carriage in Poland was from a study on 20 healthy children in 2002 where one was positive for ESBL-producing E. coli. The study population is too different and too small to compare the data to our results [23].
This study has limitations when it comes to study population, representativeness and comparability. The varying size of the study populations rendered large confidence intervals in some study populations (Table 1). Difference in culture and healthcare structure made it difficult to perform a study with a random sample from the population. Moreover response rate data was not available for analysis because the collection of this data either failed or was not possible due to the sampling method. Sampling was only done at a regional level and not validated for being representative for the entire country. Therefore the external validity of our results is unknown which makes it hard to estimate how well our findings reflect the general carriage in the community in partner countries. With that said, the majority of studies done on carriers to estimate community prevalence were done on similar study populations [13, 24].
Future studies of the molecular epidemiology of the isolates in the EP-EC population will bring more information on relatedness between the isolates in the studied countries. Regarding the screening plates used, ChromeID-ESBL and ChromeID-OXA-48, isolates that were resistant to only carbapenemases (non-OXA-48) with no ESBL co-resistance could have been negative in the screen. For Germany, isolates that were non-ESBL OXA-48 producers could possibly have been missed due to the use of different plates. However, the current epidemiology of carbapenemase-producing E. coli is mainly driven by plasmids which harbours co-resistance to several antibiotics, including cephalosporins, and would because of this grow on the ChromeID-ESBL plates. Therefore, we estimate that no, or very few, carbapenemase-producing isolates were missed because of the screening strategy.
Only the age exclusion criteria were enforced in Germany, which was a bias compared to other countries study populations. However, this did not contribute to an increased prevalence in Germany (2.3%) and neither did the higher travel frequency (due to that a vaccination clinic was used for the recruitment).
To conclude, our results should be regarded as a first insight into the prevalence of ESBL-carriers in this region, but more studies are needed to get a comprehensive understanding of the situation in partner countries. Surveillance of resistance in carriers is an important complement to the surveillance of clinical isolates since it measures the penetration of antibiotic resistance in society. It helps us to elucidate potential dissemination routes and what sectors that contribute to the increasing resistance seen for clinical isolates.
References
Van Aken S, Lund N, Ahl J, Odenholt I, Tham J (2014) Risk factors, outcome and impact of empirical antimicrobial treatment in extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia. Scand J Infect Dis 46(11):753–762. https://doi.org/10.3109/00365548.2014.937454
Freeman JT, McBride SJ, Nisbet MS, Gamble GD, Williamson DA, Taylor SL, Holland DJ (2012) Bloodstream infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae at a tertiary care hospital in New Zealand: risk factors and outcomes. Int J Infect Dis 16(5):e371–e374. https://doi.org/10.1016/j.ijid.2012.01.008
Rottier WC, Bamberg YR, Dorigo-Zetsma JW, van der Linden PD, Ammerlaan HS, Bonten MJ (2015) Predictive value of prior colonization and antibiotic use for third-generation cephalosporin-resistant Enterobacteriaceae bacteremia in patients with sepsis. Clin Infect Dis 60(11):1622–1630. https://doi.org/10.1093/cid/civ121
Ojer-Usoz E, Gonzalez D, Vitas AI (2017) Clonal diversity of ESBL-producing Escherichia coli isolated from environmental, human and food samples. Int J Environ Res Public Health 14(7). https://doi.org/10.3390/ijerph14070676
Borjesson S, Ny S, Egervarn M, Bergstrom J, Rosengren A, Englund S, Lofmark S, Byfors S (2016) Limited dissemination of extended-spectrum beta-lactamase- and plasmid-encoded AmpC-producing Escherichia coli from food and farm animals, Sweden. Emerg Infect Dis 22(4):634–640. https://doi.org/10.3201/eid2204.151142
Kluytmans JAJW, Overdevest ITMA, Willemsen I, Kluytmans-van den Bergh MFQ, van der Zwaluw K, Heck M, Rijnsburger M, Vandenbroucke-Grauls CMJE, Savelkoul PHM, Johnston BD, Gordon D, Johnson JR (2013) Extended-spectrum β-lactamase–producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis 56(4):478–487. https://doi.org/10.1093/cid/cis929
ECDC/EFSA/EMA (2017) ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J 15(7)
Ben-Ami R, Rodriguez-Bano J, Arslan H, Pitout JD, Quentin C, Calbo ES, Azap OK, Arpin C, Pascual A, Livermore DM, Garau J, Carmeli Y (2009) A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis 49(5):682–690. https://doi.org/10.1086/604713
Tängdén T, Cars O, Melhus Å, Löwdin E (2010) Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum β-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother 54(9):3564–3568. https://doi.org/10.1128/aac.00220-10
Kantele A, Laaveri T, Mero S, Vilkman K, Pakkanen SH, Ollgren J, Antikainen J, Kirveskari J (2015) Antimicrobials increase travelers' risk of colonization by extended-spectrum betalactamase-producing Enterobacteriaceae. Clin Infect Dis 60(6):837–846. https://doi.org/10.1093/cid/ciu957
Lübbert C, Straube L, Stein C, Makarewicz O, Schubert S, Mössner J, Pletz MW, Rodloff AC (2015) Colonization with extended-spectrum beta-lactamase-producing and carbapenemase-producing Enterobacteriaceae in international travelers returning to Germany. Int J Med Microbiol 305(1):148–156. https://doi.org/10.1016/j.ijmm.2014.12.001
Arcilla MS, van Hattem JM, Haverkate MR, Bootsma MCJ, van Genderen PJJ, Goorhuis A, Grobusch MP, Lashof AMO, Molhoek N, Schultsz C, Stobberingh EE, Verbrugh HA, de Jong MD, Melles DC, Penders J (2017) Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis 17(1):78–85. https://doi.org/10.1016/s1473-3099(16)30319-x
Woerther P-L, Burdet C, Chachaty E, Andremont A (2013) Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev 26(4):744–758. https://doi.org/10.1128/cmr.00023-13
Valenza G, Nickel S, Pfeifer Y, Eller C, Krupa E, Lehner-Reindl V, Höller C (2014) Extended-spectrum-β-lactamase-producing Escherichia coli as intestinal colonizers in the German community. Antimicrob Agents Chemother 58(2):1228–1230. https://doi.org/10.1128/aac.01993-13
Ny S, Lofmark S, Borjesson S, Englund S, Ringman M, Bergstrom J, Naucler P, Giske CG, Byfors S (2017) Community carriage of ESBL-producing Escherichia coli is associated with strains of low pathogenicity: a Swedish nationwide study. J Antimicrob Chemother 72(2):582–588. https://doi.org/10.1093/jac/dkw419
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
ECDC (2018) European Antimicrobial Resistance Surveillance Network (EARS-Net). https://ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/data-ecdc accessed on 2018-07-06
Sukhorukova MV, Edelstein MV, Skleenova EY, Ivanchik NV, Mikotina AV, Dekhnich AV, Kozlov RS, the «MARATHON» study group (2017) Antimicrobial resistance of nosocomial Enterobacteriaceae isolates in Russia: results of multicenter epidemiological study «MARATHON» 2013-2014. KMAKH 19(1):49–56
Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchounski L (2003) Prevalence and molecular epidemiology of CTX-M extended-spectrum -lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob Agents Chemother 47(12):3724–3732
Kuzmenkov AY, Trushin IV, Avramenko AA, Edelstein MV, Dekhnich AV, Kozlov R (2017) AMRmap: an online platform for monitoring antibiotic resistance. КМАХ 19(2):84–90 http://tinyurl.com/ycujeomm accessed on 2018-07-06
Public Health Agency of Sweden and the National Veterinary Institute (2016) Swedres-Svarm; Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. https://www.folkhalsomyndigheten.se/contentassets/d118ac95c12d4c11b3e61d34ee6d2332/swedres-svarm-2016-16124.pdf accessed on 2018-07-06
Terveyden ja hyvinvoinnin laitos, Jalava J, Räisänen K (eds.) (2016) Bakteerien mikrobilääkeresistenssi Suomessa, Finres. http://urn.fi/URN:ISBN:978-952-302-958-3 accessed on 2018-07-06
Franiczek R, Sobieszczańska B, Grabowski M, Mowszet K, Pytrus T (2003) Occurrence of extended-spectrum β-lactamases among Escherichia coli isolates from hospitalized and healthy children. Folia Microbiol 48(2):243–247
Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E (2016) Fecal colonization with extended-spectrum beta-lactamase–producing Enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis 63(3):310–318. https://doi.org/10.1093/cid/ciw283
Acknowledgements
We would like to thank all participating centres, staff and volunteers that took part in the study. A special thank you to the NDPHS secretariat for financial advice and organisation as well as help with project management. Also thank you to Dr. Martin Steinbakk for advice during the set-up of the study.
NoDARS ESBL-carrier working group consists of the following persons: Kaisu Rantakokko-Jalava, Marianne Gunell, Esa Rintala, Harri Marttila, Tiina Kurvinen, Emilia Lönnqvist, Mari Virta, Anne-Mari Kaarto, Mikko Talja, Tim Eckmanns, Muna Abu Sin, Edward Velasco, Arne C. Rodloff, Felix Samuel Lempp, Norman Lippmann, Natali Ivanchik, Oliver Dyar, Christian Giske, Piotr Z. Brewczyński, Lidia Horoń, Anu Kantele and Sari H. Pakkanen.
Funding
This study was funded by the European Commission (grant contract number 2014/344-660) and all affiliated partner organisations. Additional funding was received from the German Federal Ministry of Health and the Finnish Ministry of Social Affairs and Health.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained for Sweden (Etikprövningsnämnden Stockholm: 2015/1893-31/1), Finland (Ethics Committee, Hospital District of Southwest Finland, :ETMK 158/1801/2015), Russia (Independent Ethics Committee of Smolensk State Medical University #184), Germany (Ethics Committee of Charité—Universitätsmedizin Berlin: EA2/008/15), Latvia (Ethics committee for clinical research at Pauls Stradins Clinical University Hospital: 040815—12E), Poland (Bioethical Commission in IOMEH: 1/2016).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ny, S., Kozlov, R., Dumpis, U. et al. Large variation in ESBL-producing Escherichia coli carriers in six European countries including Russia. Eur J Clin Microbiol Infect Dis 37, 2347–2354 (2018). https://doi.org/10.1007/s10096-018-3382-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3382-8