Abstract
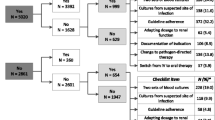
This study aimed to improve the quality of documentation on antibiotic therapy in the computerized medical records of inpatients. A prospective, uncontrolled, interrupted time series (ITS) study was conducted by repeated point prevalence survey (PPS) to audit the quality of documentation on antibiotic therapy in the medical records before and after a combined intervention strategy (implementation of guidelines, distribution of educational materials, educational outreach visits, group educational interactive sessions) from the antimicrobial stewardship team (AST) in the academic teaching hospital (CHU) of Liège, Belgium. The primary outcome measure was the documentation rate on three quality indicators in the computerized medical records: (1) indication for treatment, (2) antibiotics prescribed, and (3) duration or review date. Segmented regression analysis was used to analyze the ITS. The medical records of 2306 patients receiving antibiotics for an infection (1177 in the pre-intervention period and 1129 in the post-intervention period) were analyzed. A significant increase in mean percentages in the post-intervention period was observed as compared with the pre-intervention period for the three quality indicators (indication documented 83.4 ± 10.4 % vs. 90.3 ± 6.6 %, p = 0.0013; antibiotics documented 87.9 ± 9.0 % vs. 95.6 ± 5.1 %, p < 0.0001; and duration or review date documented 31.9 ± 15.4 % vs. 67.7 ± 15.2 %, p < 0.0001). The study demonstrated the successful implementation of a combined intervention strategy from the AST. This strategy was associated with significant changes in the documentation rate in the computerized medical records for the three quality indicators.

Similar content being viewed by others
References
European Centre for Disease Prevention and Control (ECDC) (2014) Surveillance report. Antimicrobial resistance surveillance in Europe 2013. ECDC, Stockholm. doi:10.2900/39777
Frieden T (2013) Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention (CDC), 114 pp
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB et al (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi:10.1086/595011
Van Gastel E, Costers M, Peetermans WE, Struelens MJ; Hospital Medicine Working Group of the Belgian Antibiotic Policy Coordination Committee (2010) Nationwide implementation of antibiotic management teams in Belgian hospitals: a self-reporting survey. J Antimicrob Chemother 65:576–580. doi:10.1093/jac/dkp470
Allerberger F, Mittermayer H (2008) Antimicrobial stewardship. Clin Microbiol Infect 14:197–199. doi:10.1111/j.1469-0691.2007.01929.x
Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP et al (2007) Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177. doi:10.1086/510393
Van Gastel E, Balligand E, Costers M, Magerman K; Hospital Medicine Working Group of the Belgian Antibiotic Policy Coordination Committee (2015) Antibiotic management teams in Belgian hospitals: continued improvement in the period from 2007 to 2011. Eur J Clin Microbiol Infect Dis 34:673–677. doi:10.1007/s10096-014-2279-4
Willemsen I, Groenhuijzen A, Bogaers D, Stuurman A, van Keulen P, Kluytmans J (2007) Appropriateness of antimicrobial therapy measured by repeated prevalence surveys. Antimicrob Agents Chemother 51:864–867. doi:10.1128/AAC.00994-06
Zarb P, Amadeo B, Muller A, Drapier N, Vankerckhoven V, Davey P et al (2011) Identification of targets for quality improvement in antimicrobial prescribing: the web-based ESAC Point Prevalence Survey 2009. J Antimicrob Chemother 66:443–449. doi:10.1093/jac/dkq430
Ansari F, Erntell M, Goossens H, Davey P (2009) The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis 49:1496–1504. doi:10.1086/644617
Department of Health, ESPAUR SSTF subcommittee (2015) Start smart - then focus. Antimicrobial stewardship toolkit for English Hospitals. 26 pp
Malcolm W, Nathwani D, Davey P, Cromwell T, Patton A, Reilly J et al (2013) From intermittent antibiotic point prevalence surveys to quality improvement: experience in Scottish hospitals. Antimicrob Resist Infect Control 2:3. doi:10.1186/2047-2994-2-3
Cooke FJ, Matar R, Lawson W, Aliyu SH, Holmes A (2010) Comment on: antibiotic stewardship—more education and regulation not more availability? J Antimicrob Chemother 65:598. doi:10.1093/jac/dkp481
Gloucestershire Hospitals NHS Foundation Trust (2013) Antibiotic stop/review date and indication policy, 6 pp
Cooke FJ, Holmes AH (2007) The missing care bundle: antibiotic prescribing in hospitals. Int J Antimicrob Agents 30:25–29. doi:10.1016/j.ijantimicag.2007.03.003
Cochrane Effective Practice and Organisation of Care Review Group (EPOC) (2002) Data collection checklist. Accessed 27 May 2014
Ramsay C, Brown E, Hartman G, Davey P (2003) Room for improvement: a systematic review of the quality of evaluations of interventions to improve hospital antibiotic prescribing. J Antimicrob Chemother 52:764–771. doi:10.1093/jac/dkg460
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D (2002) Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 27:299–309. doi:10.1046/j.1365-2710.2002.00430.x
Delory T, De Pontfarcy A, Emirian A, About F, Berdougo B, Brun-Buisson C et al (2013) Impact of a program combining pre-authorization requirement and post-prescription review of carbapenems: an interrupted time-series analysis. Eur J Clin Microbiol Infect Dis 32:1599–1604. doi:10.1007/s10096-013-1918-5
Fowler S, Webber A, Cooper BS, Phimister A, Price K, Carter Y et al (2007) Successful use of feedback to improve antibiotic prescribing and reduce Clostridium difficile infection: a controlled interrupted time series. J Antimicrob Chemother 59:990–995. doi:10.1093/jac/dkm014
Aldeyab MA, Kearney MP, McElnay JC, Magee FA, Conlon G, MacIntyre J et al (2012) A point prevalence survey of antibiotic use in four acute-care teaching hospitals utilizing the European Surveillance of Antimicrobial Consumption (ESAC) audit tool. Epidemiol Infect 140:1714–1720. doi:10.1017/S095026881100241X
van Spreuwel PCJM, Blok H, Langelaar MFM, Kullberg BJ, Mouton JW, Natsch S (2015) Identifying targets for quality improvement in hospital antibiotic prescribing. Neth J Med 73:161–168
Cotta MO, Robertson MS, Upjohn LM, Marshall C, Liew D, Buising KL (2014) Using periodic point-prevalence surveys to assess appropriateness of antimicrobial prescribing in Australian private hospitals. Intern Med J 44:240–246. doi:10.1111/imj.12353
Hansen S, Sohr D, Piening B, Pena Diaz L, Gropmann A, Leistner R et al (2013) Antibiotic usage in German hospitals: results of the second national prevalence study. J Antimicrob Chemother 68:2934–2939. doi:10.1093/jac/dkt292
Toth NR, Chambers RM, Davis SL (2010) Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm 67:746–749. doi:10.2146/ajhp090259
Coll A, Kinnear M, Kinnear A (2012) Design of antimicrobial stewardship care bundles on the high dependency unit. Int J Clin Pharm 34:845–854. doi:10.1007/s11096-012-9680-9
Chaves NJ, Ingram RJ, MacIsaac CM, Buising KL (2014) Sticking to minimum standards: implementing antibiotic stewardship in intensive care. Intern Med J 44:1180–1187. doi:10.1111/imj.12539
Roberts E, Dawoud DM, Hughes DA, Cefai C (2015) Evaluation of a consultant audit and feedback programme to improve the quality of antimicrobial prescribing in acute medical admissions. Int J Pharm Pract 23:333–339. doi:10.1111/ijpp.12173
Pulcini C, Defres S, Aggarwal I, Nathwani D, Davey P (2008) Design of a ‘day 3 bundle’ to improve the reassessment of inpatient empirical antibiotic prescriptions. J Antimicrob Chemother 61:1384–1388. doi:10.1093/jac/dkn113
Willemsen I, van der Kooij T, van Benthem B, Wille J, Kluytmans J (2010) Appropriateness of antimicrobial therapy: a multicentre prevalence survey in the Netherlands, 2008–2009. Eur Surveill 15. pii: 19715
Cusini A, Rampini SK, Bansal V, Ledergerber B, Kuster SP, Ruef C et al (2010) Different patterns of inappropriate antimicrobial use in surgical and medical units at a tertiary care hospital in Switzerland: a prevalence survey. PLoS One 5:e14011. doi:10.1371/journal.pone.0014011
Akhloufi H, Streefkerk RH, Melles DC, de Steenwinkel JEM, Schurink CAM, Verkooijen RP et al (2015) Point prevalence of appropriate antimicrobial therapy in a Dutch university hospital. Eur J Clin Microbiol Infect Dis 34:1631–1637. doi:10.1007/s10096-015-2398-6
Gyssens IC (2001) Quality measures of antimicrobial drug use. Int J Antimicrob Agents 17:9–19. doi:10.1016/S0924-8579(00)00208-9
Acknowledgments
We would like to thank the clinical staff involved in this study, P. Papineni for the language revision, and C. Achen for the helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No specific funding was received for this work.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Vercheval, C., Gillet, M., Maes, N. et al. Quality of documentation on antibiotic therapy in medical records: evaluation of combined interventions in a teaching hospital by repeated point prevalence survey. Eur J Clin Microbiol Infect Dis 35, 1495–1500 (2016). https://doi.org/10.1007/s10096-016-2690-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2690-0