Abstract
Varicella-zoster virus (VZV), an important member of the Herpesviridae family, is the etiological agent of varicella as primary infection and zoster as recurrence. An outstanding feature is the lifelong viral latency in dorsal root and cranial nerve ganglia. Both varicella and zoster are worldwide widespread diseases that may be associated with significant complications. However, there is a broad spectrum of laboratory methods to diagnose VZV infections. In contrast to many other viral infections, antiviral treatment of VZV infections and their prevention by vaccination or passive immunoprophylaxis are well established in medical practice. The present manuscript provides an overview about the basic knowledge of VZV infections, their laboratory diagnosis, antiviral therapy, and the prevention procedures, especially in Germany.
Similar content being viewed by others
Virus, epidemiology, and infection
Virus
Varicella-zoster virus (VZV) is a member of the genus Varicellovirus within the subfamily Alphaherpesvirinae and the family Herpesviridae. Varicella-zoster virus is an enveloped DNA virus with low environmental resistance and has a size of 150–200 nm [1]. The viral genome consists of double-stranded DNA with a length of 125 kb and comprises 73 genes, 70 of which are unique and three are duplicated. The icosahedral capsid has 162 capsomers and is surrounded by a lipid envelope comprising of host cell components and virus-encoded glycoproteins. Between the nucleocapsid and the envelope, the tegument is located as a protein layer. The virus binds via glycoproteins to cellular receptors like the mannose-6-phosphate and penetrates the cellular membrane thereafter. As with all herpesviruses, the viral replication is a complex cascade-adjusted process with sequential expression of α, β, and γ genes, mainly taking place within the cellular nucleus. Varicella-zoster virus has only one serotype. Despite a pronounced genetic homogeneity, there are nucleotide polymorphisms within the VZV genome leading to the classification of five major clades after whole genome sequencing, showing different geographical distributions [2]. The VZV DNA has numerous sequence homologies with the herpes simplex virus (HSV) genome. This fact has to be considered if primers are selected for the diagnostic amplification of viral DNA. Likewise, serological cross-reactions with HSV are also of diagnostic relevance, most likely reflecting common antigenic determinants of viral glycoproteins.
Epidemiology
Varicella-zoster virus is distributed worldwide in humans. The virus is highly contagious and is transmitted predominantly by airborne droplet infection. In many cases, humans are the source of infection at the end of the incubation period after primary infection. Infected individuals excrete the virus via saliva or conjunctival fluid from two days before the onset of varicella exanthema. The fluid of skin vesicles is also highly infectious before the lesions are completely encrusted. In case of zoster, the risk of spreading the infection is significantly lower, since, in most cases, only the vesicle fluid is infectious. While in countries with temperate climate the majority of children develop varicella before the age of 10 years, a relatively small proportion of children in tropical and subtropical areas have been demonstrated to be VZV-seropositive, and varicella has been shown to affect mainly adolescents and adults [3]. Before the implementation of universal varicella vaccination in Germany in 2004, VZV seroprevalence showed a rapid increase during the first decade of life and reached between 80 % and more than 90 % [4]. Among the people more than 40 years old, only isolated individuals were susceptible to VZV. In women of child-bearing age, VZV seroprevalence is calculated as approximately 95–97 %. Risk groups for life-threatening primary VZV infections are seronegative adults, young infants from seronegative mothers, patients with immunodeficiency, unborn children in case of maternal varicella during the first 4–5 months of gestation, and newborns from mothers with varicella infections shortly before or after delivery. There is an increased risk of zoster in elderly people, immunodeficient patients, and children after varicella during pregnancy or the first year of life. Studies for VZV genotyping have provided new insights into the geographic distribution and phylogenetic analysis of different VZV clades. In Europe, the European clades 1 and 3 are distributed, but the African clade 5 also occurs due to increasing migratory movements. Clade 2 represents the dominant clade in Asia, and clade 4 has rarely been observed on different continents until now [5].
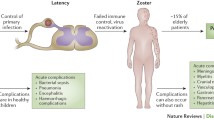
Latency
Varicella-zoster virus is cytopathogenic during productive infection. However, after primary infection, it can establish latency in ganglion cells. Following centripetal axonal transport, circular viral DNA persists in neurons of dorsal root and cranial nerve ganglia, where it can remain quiescent for years or even decades, respectively. From there, viruses may be reactivated and may cause recurrent infections, called zoster, after centrifugal transport via nerve axons. The cumulative incidence of VZV reactivations leading to zoster increases significantly in older people [6], since waning VZV-specific cell-mediated immunity is an important contributor to susceptibility to zoster [7]. Asymptomatic recurrent infections may occur, but their prevalence is unknown. During latency, there is evidence for a restricted transcription of the VZV genome, and immediate early as well as early proteins from several open reading frames (ORF 4, 21, 29, 62, and 63) can be detected in human neurons. In particular, the expression of ORF 63 is a characteristic marker of VZV latency [8]. While the viral gene products occur exclusively in the cytoplasm of neurons during latency, they can also be detected in cell nuclei during virus reactivation. Currently, it is assumed that there is a continuous but low-level viral replication under immunologic control during VZV latency [9]. A reduction of the immune response as in older people or immunocompromised patients may lead to viral reactivation. It is also conceivable that the cytoplasmatic restriction of viral proteins prevents the DNA replication in cell nuclei. Recurrent infections might be triggered by the abolition of this restriction, followed by the activation of virus replication in the nucleus and virus cell-to-cell transmission.
Pathogenesis
During primary infection (incubation period 10–21 days), VZV invades the body through the mucous membranes of the upper respiratory tract and undergoes the first phase of replication in the regional lymph nodes. It follows a primary lymphocyte-associated viremia 4–6 days post infectionem (p.i.). By doing so, the virus infects peripheral blood mononuclear cells, followed by a secondary viremia 10–14 days p.i. disseminating the virus to the skin [10]. Infection spreads from the endothelial cells of the skin capillaries to the epithelial cells and initiates a local inflammatory response with the formation of vesicles by the accumulation of tissue fluid. After fusion of infected cells, multinucleated giant cells with eosinophilic inclusion bodies arise. A recurrent infection occurs always after endogenous reactivation of VZV that establishes lifelong latency after primary infection.
Diseases
Varicella/chickenpox
In more than 95 %, the primary VZV infection results in varicella [11]. In temperate climates, the disease peaks during winter and early spring. Local epidemics can occur at intervals of 3–4 years. Before the universal varicella vaccination was introduced, approximately 750,000 varicella cases per year were observed in Germany [12]. The clinical pictures range from harmless varicella during childhood to severe courses in immunodeficient patients of all age groups. Without prodromes, the disease begins suddenly with an itchy rash and, in one-third of patients, with moderate fever. Varicella exanthema is characterized initially by pinhead to pea-sized erythematous macules developing consecutively to papules, water-clear vesicles, yellowish pustules, and crusts. During uncomplicated courses, the lesions heal without any scars. Since areas of new lesions appear over a range of 4–5 days, there are always different stages of exanthema simultaneously. These result in the picture of a “starry sky” and are a characteristic feature for the differential diagnosis of varicella. As a rule, the contagiosity of varicella ends approximately 5–7 days after the onset of exanthema with complete crusting of skin vesicles. After about 2 weeks, the exanthema is completely healed.
Varicella complications have rarely been observed in immunocompetent preschool children [13]. However, the disease is a special risk for patients with impaired cellular immune function, e.g., patients with oncological diseases, organ or bone marrow transplantation, autoimmunopathies, congenital immune defects, or individuals infected with the human immunodeficiency virus [14]. The most common complications are those attributable to secondary bacterial infections, and neurological and hematological manifestations. In addition, varicella during pregnancy is associated with a high risk of maternal pneumonia and congenital transmission of the virus, leading to severe fetal sequelae [15]. Varicella pneumonia has been considered the most important complication in pregnant women. After varicella infection between 5 and 24 gestational weeks, a congenital varicella syndrome with 30 % mortality can be expected in 1–2 % of the cases. The main clinical symptoms are segmental cicatricial skin lesions, neurological diseases, eye diseases, and limb hypoplasia. In case of maternal varicella between 5 days before and 2 days after delivery, there is a high risk of virus transmission to the infant by transplacental, contact, or droplet infection. Since these infants have not yet acquired protecting maternal antibodies, generalized neonatal varicella with fatal outcome may arise in up to 20 % of the cases when the disease is not treated with acyclovir.
The repeated occurrence of varicella, so-called secondary varicella, is almost exclusively observed in patients with impaired cellular immune response. In immunocompetent people, exogenous reinfections mostly show an asymptomatic course [16]. Breakthrough diseases can be considered as a new form of varicella caused by the wild-type virus and occurring at the earliest at 42 days after single varicella vaccination. The prevalence has been calculated as 4 (−9) % of the persons vaccinated annually [13]. Most breakthrough diseases are very mild. With less than 50 skin lesions and only maculopapular lesions in about 50 % of the cases, the infectivity is relatively low and there is also only a low or even no risk for complications at all [17].
Herpes zoster/shingles
Herpes zoster, also referred shortly as zoster, always reflects a recurrent VZV infection after endogenous virus reactivation. In Germany, zoster prevalent with more than 400,000 cases per year is one of the most common viral skin infections (neurocutaneous disease) [18]. The study group for varicella at the Robert Koch-Institut (Berlin, Germany) has reported an increasing frequency of zoster, especially in people aged over 50 years, during the last several years [19]. However, this trend began before the universal varicella vaccination was recommended in 2004.
Zoster is preceded by a prodromal phase consisting of uncharacteristic mild general symptoms for 2–5 days. Typical symptoms are burning pains and/or sensory disturbances in the area of one to three adjoining dermatomes. The disease begins with skin erythema, followed by characteristic grouped papules developing to vesicles. The formation of skin vesicles lasts for 1–5 days. Afterwards, the vesicles dry out over 7–12 days, and zoster has healed in immunocompetent persons after 2–4 weeks. By contrast, the disease can follow a chronic course accompanied by skin lesions persisting for months and occurring repeatedly in immunodeficient patients. Zoster is predominantly localized in thoracic skin regions. With increasing age, the innervation areas of the trigeminal nerve are affected. In immunocompromised patients, zoster disease is more severe and more frequently associated with complications in principle. The most important complications of zoster are neurological manifestations, hemorrhagic and necrotic skin changes, bacterial superinfections, disseminations of infection, and inclusion of eyes or ears [20]. Due to zoster ganglionitis, accompanying meningitis can occur. Pains lasting longer than 4 weeks and occurring again after a pain-free interval are designated as postzosteric neuralgia caused by an irreversible necrosis of ganglion cells. Risk factors are advanced age, dermatome-associated pain during prodromal phase, female sex, more than 50 efflorescences in the affected dermatome, hemorrhagic efflorescences, and manifestations of cranial or sacral dermatomes [21]. The following possible pathomechanisms of postzosteric neuralgia are under discussion: peripheral sensitization of nociceptive C fibers followed by central sensitization of spinal nociceptive neurons, and degeneration of nociceptive C fibers as a result of inflammation. Zoster during pregnancy generally does not cause fetal sequelae.
Laboratory diagnosis
Submission of samples
Varicella-zoster virus-positive samples have to be considered as dangerous goods of category B, risk group 2, and have to be shipped according to UN 3373 regulations. To this end, the primary container containing the patient’s sample must be shipped with an outer packaging containing adsorbing material in a transport box (cardboard box). Shipment is possible at room temperature, and cooling is only recommended if samples are intended for virus isolation in cell culture [22].
Detection of virus
Acute VZV infection is diagnosed by detection of the virus (Table 1). The method of choice is polymerase chain reaction (PCR) to detect viral genomes in vesicle fluids, cerebrospinal fluids, tissues, bronchoalveolar lavages, EDTA blood, or amniotic fluids [23]. Polymerase chain reaction has specific importance for testing cerebrospinal fluids in case of suspected acute infections of the central nervous system (CNS) [24], as well as for testing amniotic fluids within prenatal diagnostics after varicella during pregnancy [22]. In immunosuppressed patients with zoster, detection of VZV DNA in blood may be helpful to verify the potential risk for dissemination of infection. Isolation of VZV is only possible in a few cell types, such as human embryonic fibroblasts. This procedure is time-consuming, requires a high degree of experience, and has no clinically relevant sensitivity. In most cases, only vesicle fluids containing high virus load are suitable for viral isolation. For successful viral isolation, an early and careful procurement as well as optimal transport of samples are essential. Identification of viral isolates is carried out properly by immunofluorescence using monoclonal fluorescein-labeled antibody. Direct qualitative detection of VZV antigens by the use of commercial detection systems may provide results within a few hours, but is characterized by reduced sensitivity and specificity. For the interpretation of results, it must be remembered that methods for direct detection of VZV, including nucleic acids or antigens, do not allow any differentiation between primary and recurrent infection. Discrimination between VZV wild-type and vaccine strains can be performed by restriction fragment length polymorphism analysis and sequencing (genotyping), respectively [25, 26].
Detection of antibodies
Serological VZV diagnosis (Table 2) is especially indicated if susceptible persons have to be identified to initialize active or passive immunoprophylaxis. Because of the high rates of seroconversion, the determination of antibody status is not necessary after varicella vaccination in healthy children, adolescents, and adults. By contrast, the control of immune status is recommended for immunodeficient vaccinees and healthcare workers [27]. In the daily laboratory practice, ligand assays or partial immunofluorescence tests are common for the determination of VZV-specific IgG antibodies. Regardless of the test used, each result interpreted as anti-VZV IgG-positive by the respective laboratory can be used as criterion for immunity against varicella. Individuals with borderline findings should be classified as “not immune“. Commercially available test kits differ with respect to sensitivity, so that very low antibody titers are not recognized. Therefore, highly sensitive tests such as special glycoprotein enzyme-linked immunosorbent assays (ELISAs) or the fluorescence antibody to membrane antigen (FAMA) test should be used to control immune status after varicella vaccination and for vaccine studies [28, 29]. The laboratory diagnosis of VZV primary infection (varicella) can be realized by the determination of VZV IgG seroconversion. To this end, there is a need to obtain sequential blood samples, of which the initial sample has to be anti-VZV IgG-negative. Anti-VZV IgM will be detectable, usually in combination with anti-VZV IgG, at the earliest from the fourth day after the onset of disease. Even though anti-VZV IgM is commonly used in practice to confirm active VZV infection, it has to be kept in mind that IgM antibodies will be detectable with significant delay after the onset of varicella exanthema and only in maximally 50–60 % of patients with zoster [23]. In addition, numerous commercial VZV IgM immunoassays have a reduced sensitivity and may show false-positive results caused by cross-reactions with other herpesviruses, in particular with HSV [15]. Especially in case of positive anti-VZV IgM without virus detection in pregnant women, false-positive test results should be excluded by repeating the test and the use of alternative test kits [22]. Anti-VZV IgA may be determined frequently in persons infected latently with VZV, but high titer values exclusively correlate with zoster disease. Intrathecal VZV-specific IgG antibodies may be of significance for the retrospective diagnosis of VZV-associated CNS infections [24]. Determination of VZV IgG avidity allows the differentiation between primary (varicella) and recurrent infections (zoster), but there is only limited experience in this capacity [30].
Antiviral therapy
Antiviral agents in clinical use
Replication of VZV in infected cells can be blocked by the administration of antiviral agents. An early administration, especially in zoster, may reduce the damage of tissue, and, thus, the destruction of affected ganglion cells can be diminished or even prevented. Primarily, the acyclic nucleoside analogs acyclovir, including its prodrug valaciclovir, famciclovir (prodrug of penciclovir), and the cyclic nucleoside analog brivudin [(E)-5-(2-bromovinyl)-2′-deoxyuridine, BVDU] are available for the antiviral treatment of VZV infections (Table 3). The specificity of antiviral activity is based on the fact that these inhibitors are phosphorylated by the viral thymidine kinase (TK) to their mono- (acyclovir, penciclovir) or diphosphates (brivudin), while the further phosphorylation steps to the triphosphate are catalyzed by cellular enzymes. Thus, the spectrum of activity is defined by the presence of the key enzyme, the viral TK. The triphosphates of the nucleoside analogs inhibit and fix the viral DNA polymerases (pol) and are incorporated as “false” substrate into the growing DNA chain. In case of acyclovir/valaciclovir, this results in chain termination due to the absence of the hydroxy group in the 3′ position essential for further linking. In other nucleoside analogous compounds, their incorporation into DNA is possible.
Acyclovir
Acyclovir is the standard therapeutic agent for the antiviral treatment of VZV infections. However, it should be considered that the oral bioavailability is only 15–30 %. Varicella in risk patients and zoster disease in immunocompetent patients can be treated orally. In severe VZV infections, especially in immunodeficient patients, acyclovir has to be administered intravenously (i.v.). After i.v. administration of acyclovir, side effects on the CNS have been observed occasionally, whereas the oral medication can be associated with gastrointestinal side effects. Substances with kidney toxicity should not be combined simultaneously with acyclovir. Laboratory kidney and liver parameters have to be monitored.
Valaciclovir
The prodrug (L-valyl ester) of acyclovir is administered orally. After oral uptake, valaciclovir is converted into acyclovir by a hepatic enzyme, the valaciclovir hydrolase. Valaciclovir has an oral bioavailability of 54 %, resulting in three to four times higher drug concentrations than after oral uptake of acyclovir. The consequences are longer dosing intervals and a better compliance. The administration of valaciclovir is approved for the antiviral treatment of zoster in immunocompetent adults. Valaciclovir is not approved for antiviral treatment during childhood and adolescence. Possible side effects are similar to those after medication of acyclovir.
Famciclovir
Famciclovir is the inactive diacetyl ester prodrug of penciclovir, arising by the separation of two ester groups in the small intestine and liver. Penciclovir is an acyclic nucleoside analog (exchange of the ether oxygen atom in the acyclic side chain by a methyl bridge) derived from ganciclovir. The oral absorption of penciclovir is very low. That is why this drug is only used for the topic antiviral treatment of local HSV infections. After oral administration, famciclovir has a bioavailability of 77 %. It is used for antiviral therapy of zoster in immunocompetent adults and immunosuppressed patients from the age of 25 years. Similar to valaciclovir, famciclovir is not approved in childhood and adolescence. In rare cases, the taking of famciclovir can lead to headaches, mental confusion, and nausea.
Brivudin
The cyclic nucleoside analog brivudin is converted to its mono- and diphosphate by the viral TK. Brivudin is administered orally and has a bioavailability of approximately 40 %. It is used for the antiviral treatment of zoster in immunocompetent adults. Since the safety profile is unknown due to the lack of studies, brivudin is not approved for antiviral therapy in children and adolescents. Therefore, the risk–benefit ratio should be examined carefully before the agent will be used in children and adolescents, and the parents have to be informed (off-label use). In principle, brivudin is well tolerated. Nevertheless, gastrointestinal disturbances, impairment of renal function, increasing of liver enzymes, and reversible changes of blood count may occur. A simultaneous administration of 5-fluorouracil or other 5-fluoropyrimidines results in an enhanced and possibly dangerous toxicity.
Foscarnet (trisodium phosphonoformate)
The pyrophosphate analog foscarnet inhibits the viral DNA pol of numerous DNA and RNA viruses by the prevention of pyrophosphate exchange. Since foscarnet is not required to be metabolized for its antiviral activity, it also has an effectiveness against TK-negative VZV strains that are resistant to nucleoside analogs. For this reason, foscarnet is recommended for alternative antiviral treatment when there is a suspected clinical resistance to acyclovir, especially in rare cases of immunosuppressed patients with severe zoster courses. Disturbances of renal function and toxic caused ulcers of urogenital mucosa have to be considered as important side effects of foscarnet.
Development of resistance
The resistance of VZV against antiviral drugs such as acyclovir or foscarnet occurs rarely and has only been described in the literature in immunodeficient patients suffering from zoster, e.g., in the acquired immune deficiency syndrome or under immunosuppression due to cancer diseases or transplantation [31–33]. For the development of resistance, the impaired immune response and long-term administration of drugs in the context of antiviral treatment or chemoprophylaxis are of crucial significance. The weakened immune response leads to a longer virus replication, and resistant virus mutants can occur more often by an increased number of natural spontaneous mutations. Resistant viruses are selected under antiviral treatment and cannot be eliminated by the impaired immune response [34]. Generally, resistances are associated with non-synonymous mutations localized within the gene of the respective target molecule or within the gene of proteins responsible for the metabolization or the effectiveness of antiviral agents. For acyclovir and related nucleoside analogs, resistance is based almost exclusively on non-synonymous mutations of the TK gene (UL36) and rarely, mostly in connection with resistance against foscarnet, on amino acid (aa) changes in the DNA pol gene (UL28) [35]. In contrast to TK, which is not required for the replication of VZV, the DNA pol is an essential enzyme within the viral replication cycle. According to previous findings, VZV strains resistant to acyclovir due to mutations in the TK gene are always cross-resistant to brivudin [33]. To date, there are only a few studies in the literature in which the significance of TK and DNA pol mutations was verified by phenotypic findings. The main reason for this is that VZV can only be isolated rarely in cell culture from patient samples, at best, from the content of vesicles.
Thymidine kinase gene
The thymidine kinase gene has a size of 1026 bp and is coding for 342 aa. This gene contains two conserved regions: one nucleotide (adenosine triphosphate)-binding site (aa 12–29) and one nucleoside (substrate)-binding site (aa 129–145). Contrary to TK of HSV-1 and HSV-2, only a low number of natural polymorphisms have been identified in the VZV TK, but it is important to differentiate each of them from resistance mutations [33]. Compared to the VZV strain Dumas (GenBank accession no. X04370.1, clade 1), which is frequently used as a reference, all European wild-type strains comprise the aa polymorphism Serine288Leucine.
Comparably with HSV [36], the resistance mutations of the VZV TK gene are assigned to three phenotypes:
-
TK negative (TK−, no TK activity detectable, occurs most frequently)
-
TK reduced (TKr, diminished TK activity, 1–15 % of normal activity)
-
TK altered (TKa, altered TK substrate specificity, no phosphorylation of acyclovir and other nucleoside analogs)
Resistance to acyclovir may be caused by stop codons, frameshifts (deletions or insertions), or aa substitutions inside and outside of conserved gene regions. Since only a few validated resistance mutations have been reported in the literature to date, novel or unknown mutations must always be expected when clinically or phenotypically resistant VZV strains are analyzed genotypically.
DNA polymerase gene
This gene, with a size of 3585 bp, is coding for 1195 aa. There are eight conserved regions with designations I to VII and A. Similar to the TK gene, only a small number of natural polymorphisms have been reported for DNA pol [33]. Resistance-related aa substitutions are localized mostly in conserved gene centers. So far, little research has been done on natural polymorphisms and resistance mutations of the VZV DNA pol gene.
Resistance testing
In case of VZV infections, an antiviral treatment failure is assumed if there are no clinical improvements detectable during the administration of the antiviral medication, mostly acyclovir within 10 (−21) days [32, 35, 37]. This means that there is reason to suspect resistant viral strains. In these cases, an alternative treatment with foscarnet is indicated. In parallel, genotypic resistant testing and, if a viral isolate can be established in cell culture, phenotypic resistant testing should be carried out. Because of the high expenditure of time (at least 3 weeks), the phenotypic resistance testing has mostly no clinical relevance, but the procedure can help to characterize novel mutations or gene variations which are, so far, unclear with respect to their significance for any resistance.
Phenotyping
Phenotyping has been considered as the gold standard for resistance testing of VZV, but it is mostly not feasible since the VZV isolation in cell culture has low sensitivity. The plaque reduction assay has been established as the method of choice [33]. After adding the antiviral compound to be tested in descending dilution series, the 50 % effective concentration (EC50) is estimated, inducing a 50 % inhibition of viral replication. For the evaluation of possible resistance, a susceptible VZV reference strain has to be tested in each experiment as a control. It is a crucial advantage that phenotyping allows an unambiguous interpretation of the results. However, the procedures used are time- and material-consuming, as well as non-standardized. In practice, phenotypic resistance testing can only be realized if swabs can be obtained from vesicle fluids from which the virus can be isolated in cell culture. For interpretation of the results, the most common and reliable procedure for nucleoside analogs is to classify VZV strains as resistant if the mean EC50 measured is three to five times higher than the corresponding value of the sensitive control strain [38]. For resistance to foscarnet, EC50 values >300 μM have been proven to be sound [39].
Genotyping
By analogy with HSV, genotyping resistance testing of VZV is performed by means of amplification and sequencing TK and DNA pol genes [36, 40]. For the identification of non-synonymous mutations, sequence data must be compared with the published sequences of a susceptible reference strain available in the GenBank (e.g., VZV strain Dumas, accession no. X04370.1). The advantage of genotyping is a considerably shorter delay (approximately 2 days) in comparison to phenotyping and the direct testing of patient samples. The restricted quantity of viral DNA may have a limiting effect. A great disadvantage is the fact that there is only little information available about assured resistance-associated aa substitutions. That is why only stop codons or frameshift mutations can be interpreted without doubt in relation to resistance. In addition, the analysis of genotypic resistance may be difficult when a mixture of viral strains with different genotypes is present. Therefore, it is a problem in clinical practice to define a questionable resistance of VZV strains on the basis of genotyping results alone. Recently, it has become apparent that phenotypic testing of recombinant VZV isolates is the best method to validate the significance of mutations for any resistance [41].
Prevention
Prevention of varicella by vaccination
All available varicella vaccines are live attenuated vaccines and based, with exception of one non-Oka vaccine (Suduvax™, Green Cross, South Korea) [42], on the VZV Oka strain [43]. This virus strain (parental Oka, pOka) was isolated in the early 1970s from the vesicle fluid of a 3-year-old child with varicella whose surname Oka was used for the designation of this strain [44]. Varicella vaccines induce both humoral as well as T cell immunity. The seroconversion rate has been used as criterion for the assessment of vaccine immunity, which means the presence of anti-VZV IgG 6–8 weeks post-vaccination in persons being seronegative pre-vaccination. In healthy persons, the rate of seroconversion has been calculated to be more than 95 % after a single vaccine dose and 98–99 % after two doses, whereas in risk patients, the rates amount to 80–90 % [13, 45, 46]. However, the clinical effectiveness has been estimated to be partly lower, depending on the time period post-vaccination [47, 48]. In summary, vaccine efficacy ranged, in healthy children, from 80 to 97 % and from 93 to 96 % for the first and the second doses, respectively. After universal varicella vaccination was implemented in the United States in 1995 [49], the incidence of varicella was reduced by 90 % up to the year 2008 [50]. With regard to all age groups, the incidence of varicella decreased to 71–84 % until the year 2000, and hospitalizations due to varicella declined by three to four times [51]. In the monitored regions, the average immunization rates in children amounted to approximately 80 %. The pronounced herd immunity also led to the reduction of varicella incidence in unvaccinated persons. Studies suggest that the vaccine-induced antibodies persist for decades in a high percentage of the vaccinees [52, 53]. As a consequence of decreasing or insufficient immunity after vaccination, a breakthrough disease may occur in case of massive virus exposure. Despite the high rates of immunization in the United States, varicella outbreaks in schools and day care centers, as well as breakthrough diseases in vaccinated persons, have been reported [54, 55]. It was noted that there is only sufficient protection if the varicella vaccine is given twice. This knowledge has led to the introduction of a two-dose vaccination scheme for the administration of varicella vaccines [56]. A shift of varicella to higher age is not to be expected in connection with immunization rates, which are comparable to Germany. Reservations against the varicella vaccination are mostly justified by the scenario that a decline of varicella incidence may result in a decreased circulation of wild-type viruses, and, thereby, older people fall ill with zoster. In the United States, an impact of varicella vaccination on the incidence of zoster could not be observed in adults [57]. However, it has been shown that zoster is 3 to 12 times rarer in children vaccinated against varicella than in unvaccinated children [58, 59]. The disease tends to a clinically milder course, and the exanthema is frequently found anatomically close to the previous injection site of the vaccine. In rare cases, the varicella vaccine virus may cause zoster in previously vaccinated persons [60].
In July 2004, the universal varicella vaccination has been included in the vaccination schedule for all children and adolescents in Germany [61]. Up to 2011, the immunization rates increased to nearly 70 % [62]. Initially, a single vaccination using monovalent varicella vaccines was recommended for children aged between 1 and 13 years. Since 2009, the recommendation of the Standing Committee on Vaccination at the Robert Koch Institute (STIKO, Berlin, Germany) addresses a two-dose schedule as standard for varicella vaccination [63]. In subsequent years, this recommendation has been implemented without any reduction in immunization rates [62]. The first dose is administered at the age of 11–14 months, either simultaneously combined with the first measles–mumps–rubella (MMR) vaccination or, at the earliest, 4 weeks after that. Alternatively, a combined measles–mumps–rubella–varicella (MMRV) vaccine can be used. The second dose of varicella vaccination is recommended at the age of 15–23 months. Since an increased risk of febrile seizures has been observed after the first vaccination using the combined MMRV vaccine [64], a separate administration of MMR vaccine on the one hand and the varicella vaccine on the other hand has to be preferred according to the information currently available. For unvaccinated 5- to 17-year-olds without varicella history, a catch-up vaccination using two-dose varicella or MMRV vaccination is recommended. The minimum time between two doses of the varicella or MMRV vaccine should be 4–6 weeks. The varicella vaccine is recommended as indication immunization for all persons summarized in Table 4. Currently, the monovalent varicella vaccines Varilrix® from the manufacturer GlaxoSmithKline and Varivax® from Aventis Pasteur MSD, as well as the quadrivalent MMRV vaccine Priorix-Tetra® from GlaxoSmithKline, are available in Germany. All vaccines are administered subcutaneously. After contact with risk persons, the recommendations advise the varicella vaccination as post-exposure prophylaxis for non-vaccinated persons without varicella history [65]. The vaccination should be provided within 3 days after the onset of exanthema in the index case or within 5 days after contact with the index case. Sentinel results of the working group “Varicella” at the Robert Koch Institute show a decline of varicella incidence by 80–90 % up to 2012 [19], whereby all age groups have been affected. However, such age groups, for which the vaccine recommendations are applicable (1–4-year-olds) or the vaccination is highly effective (5–9-year-olds), are mostly affected. Importantly, infants susceptible for varicella benefit from herd immunity as well.
Important contraindications of varicella vaccination are intensive immunosuppressive therapy and pregnancy, including the period 4–6 weeks before a planned pregnancy. However, according to the present state of knowledge, there is no identifiable risk for prenatal malformations if varicella vaccination is performed accidentally during or shortly before pregnancy [66]. In very rare cases, transmission of vaccine virus from immunocompetent vaccinated individuals to susceptible contact persons has been reported [67]. This is, in principle, possible if the vaccinee develops exanthema with vesicles. By contrast, there may be a higher risk for virus transmission in immunodeficient patients suffering from varicella caused by the vaccine virus. Thus, individuals with risk for severe varicella, including pregnant women and newborns from mothers without varicella history, should avoid contact with vaccinees. However, the pregnancy of a mother is not considered as a contraindication for the vaccination of her unprotected child [68]. Following varicella vaccination, side effects such as redness and swelling at the injections site in approximately 4 % and pains at the injection site may occur in about 20 % of the vaccinees [66]. Among vaccinated children, 3–5 % develop skin efflorescences localized at the injection site. In a further 3–5 % of children, less pronounced varicella-like exanthemas caused by the vaccine virus can occur 2–6 weeks after vaccination, but adolescents and adults are affected twice as frequently as children. Varicella-like exanthemas within 2 weeks after vaccination are mostly caused by wild-type virus infections. Exanthemas occurring later are generally related to the vaccine virus. An unintentional vaccination of seropositive persons is not associated with an increasing number of side effects.
Passive immunoprophylaxis of varicella
Passive VZV immunoprophylaxis using varicella-zoster immunoglobulin (VZIG) may prevent the onset of varicella or weaken substantially the course of disease. Therefore, the administration of VZIG has been recommended for susceptible risk patients after exposure to varicella or zoster. This includes the following groups of individuals [65]:
-
Unvaccinated pregnant women without varicella history
-
Immunodeficient patients whose immune status to varicella is unknown or negative
-
Newborns whose mothers develop varicella within 5 days before and 2 days after delivery
-
Premature infants from 28 weeks gestation, whose mothers have no immunity, after exposure during the neonatal period
-
Premature infants younger than 28 weeks gestation after exposure during the neonatal period, regardless of the maternal immune status
If non-vaccinated pregnant women without varicella history are immunized passively, it has to be considered that the main reason for the administration of immunoglobulin is to protect pregnant women against varicella with a severe course. To date, there is no evidence that this cost-intensive approach prevents the spread of fetal disease in the form of congenital varicella syndrome [69]. However, one should be aware that the administration of VZIG is the only chance to prevent the congenital varicella syndrome after viral exposure of an unprotected woman during the first 20 weeks of gestation.
An important precondition to expect an effectiveness of passive immunoprophylaxis is the timely administration of VZIG within 3 days and maximally 10 days after beginning the exposure [65]. An “exposure“ has been defined as:
-
One hour or longer together with infectious person in one room,
-
face-to-face contact or
-
household contact.
For administration and dosing of VZIG, the manufacturers’ instructions have to be observed. If appropriate, the post-exposure administration of VZIG can be combined with antiviral chemoprophylaxis.
Prevention of zoster by vaccination
The age-dependent increase of zoster incidence is correlated with the decrease of specific T cell immunity [70]. Therefore, attempts have been made to stimulate the specific cellular immunity in elderly people and, thus, to allow the prevention of zoster. Inspired by the success of varicella vaccine, a zoster vaccine containing an at least 14 times higher concentration of the vaccine virus has been developed. It could be shown in a large study that the vaccination of adults may result in a reduction of incidence and severity of zoster by approximately 50 %, as well as the frequency of postzosteric neuralgia by 67 % [71]. According to the present information, protective immunity persists for at least 7 years. The vaccine is well tolerated. Side effects have to be expected only as local reactions at the injection site, such as redness, swelling, pain, and touch sensitivity. Under the trademark Zostavax®, the first vaccine for the prevention of zoster and postzosteric neuralgia was approved in 2006 in Europe for persons from the age of 50 years. Sanofi Pasteur MSD (Lyon, France) is the holder of the European marketing authorization. The vaccine is administered subcutaneously as a single dose [72]. As for all live vaccines, the zoster vaccine is contraindicated for immunosuppressed patients and pregnant women. The vaccine can be given simultaneously with the inactivated influenza vaccine, whereas it should not be administered simultaneously with the Pneumococcus vaccine. Zostavax® has been available in Germany since September 2013. Even though the zoster vaccination is a part of the public vaccination recommendations in the German states Saxonia, Thuringia, and Mecklenburg-Western Pomerania, the recommendation by the STIKO is still pending. Accordingly, the zoster vaccination is no an affordable service of the health insurance. Recently, a second zoster vaccine has been developed on the basis of VZV glycoprotein E [73], being of fundamental significance for the development of VZV-specific immunity. An adjuvant based on liposomes serves as an amplifier of immunity. The first results of clinical trials refer to an excellent effectiveness in persons aged 50 years and over.
References
Davison A, Eberle R, Hayward GS, McGeoch DJ, Minson AC, Pellett PE et al (2005) Herpesviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) Virus taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, pp 193–212
Breuer J, Grose C, Norberg P, Tipples G, Schmid DS (2010) A proposal for a common nomenclature for viral clades that form the species varicella-zoster virus: summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry, 24–25 July 2008. J Gen Virol 91:821–828
Lokeshwar MR, Agrawal A, Subbarao SD, Chakraborty MS, Ram Prasad AV, Weil J et al (2000) Age related seroprevalence of antibodies to varicella in India. Indian Pediatr 37:714–719
Wutzler P, Färber I, Wagenpfeil S, Bisanz H, Tischer A (2001) Seroprevalence of varicella-zoster virus in the German population. Vaccine 20:121–124
Schmidt-Chanasit J, Sauerbrei A (2011) Evolution and world-wide distribution of varicella-zoster virus clades. Infect Genet Evol 11:1–10
Hillebrand K, Bricout H, Schulze-Rath R, Schink T, Garbe E (2015) Incidence of herpes zoster and its complications in Germany, 2005–2009. J Infect 70:178–186
Gershon AA, Mervish N, LaRussa P, Steinberg S, Lo SH, Hodes D et al (1997) Varicella-zoster virus infection in children with underlying human immunodeficiency virus infection. J Infect 176:1496–1500
Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B et al (1996) Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci U S A 93:2122–2124
Kennedy PG, Rovnak J, Badani H, Cohrs RJ (2015) A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol 96:1581–1602
Arvin AM, Moffat JF, Redman R (1996) Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. Adv Virus Res 46:263–309
Schneweis KE, Krentler C, Wolff MH (1985) Durchseuchung mit dem Varicella-Zoster-Virus und serologische Feststellung der Erstimmunität. Dtsch Med Wochenschr 110:453–457
Sauerbrei A, Wutzler P (2004) Varicella-Zoster-Virus-Infektionen: Aktuelle Prophylaxe und Therapie, 1st edn. Uni-Med, Bremen, pp 44–53
Wutzler P, Knuf M, Liese J (2008) Varicella: efficacy of two-dose vaccination in childhood. Dtsch Arztebl Int 105:567–572
Arvin AM (1999) Management of varicella-zoster virus infections in children. Adv Exp Med Biol 458:167–174
Sauerbrei A (2007) Varicella-zoster virus infections during pregnancy. In: Mushahwar K (ed) Congenital and other related infectious diseases of the newborn. Perspectives in Medical Virology, vol 13. Elsevier, Amsterdam, pp 51–73
Quinlivan M, Breuer J (2006) Molecular studies of Varicella zoster virus. Rev Med Virol 16:225–250
Vázquez M, Shapiro ED (2005) Varicella vaccine and infection with varicella-zoster virus. N Engl J Med 352:439–440
Ultsch B, Köster I, Reinhold T, Siedler A, Krause G, Icks A et al (2013) Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ 14:1015–1026
Siedler A, Hecht J, Rieck T, Tolksdorf K, Hengel H (2013) Die Varizellenimpfung in Deutschland. Eine Zwischenbilanz mit Blick auf die Masern-Mumps-Röteln (MMR-)Impfung. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 56:1313–1320
Gross G (1997) Zoster—Manifestationsformen an der Haut, Komplikationen und Therapie. Dtsch Med Wochenschr 122:132–139
Wutzler P, Meister W (1997) Herpes zoster—Symptomatologie, demographische Daten und prognostische Faktoren. Dtsch Ärztebl 94:A1129–A1133
Sauerbrei A (2014) Windpocken (Varizellen). In: S2k-Leitlinie: Labordiagnostik schwangerschaftsrelevanter Virusinfektionen. Springer, Berlin, Heidelberg, pp 95–110
Sauerbrei A, Eichhorn U, Schacke M, Wutzler P (1999) Laboratory diagnosis of herpes zoster. J Clin Virol 14:31–36
Sauerbrei A, Wutzler P (2002) Laboratory diagnosis of central nervous system infections caused by herpesviruses. J Clin Virol 25(Suppl 1):S45–S51
Sauerbrei A, Uebe B, Wutzler P (2003) Molecular diagnosis of zoster post varicella vaccination. J Clin Virol 27:190–199
Sauerbrei A, Stefanski J, Philipps A, Krumbholz A, Zell R, Wutzler P (2011) Monitoring prevalence of varicella-zoster virus clades in Germany. Med Microbiol Immunol 200:99–107
Sauerbrei A, Wutzler P (2004) Labordiagnostik der Varizellen. Kinderärztl Prax, Sonderheft Impfen, pp 18–21
Sauerbrei A, Färber I, Brandstädt A, Schacke M, Wutzler P (2004) Immunofluorescence test for sensitive detection of varicella-zoster virus-specific IgG: an alternative to fluorescent antibody to membrane antigen test. J Virol Methods 119:25–30
Sauerbrei A, Wutzler P (2006) Serological detection of varicella-zoster virus-specific immunoglobulin G by an enzyme-linked immunosorbent assay using glycoprotein antigen. J Clin Microbiol 44:3094–3097
Kneitz RH, Schubert J, Tollmann F, Zens W, Hedman K, Weissbrich B (2004) A new method for determination of varicella-zoster virus immunoglobulin G avidity in serum and cerebrospinal fluid. BMC Infect Dis 4:33
Fillet AM, Visse B, Caumes E, Dumont B, Gentilini M, Huraux JM (1995) Foscarnet-resistant multidermatomal zoster in a patient with AIDS. Clin Infect Dis 21:1348–1349
Saint-Léger E, Caumes E, Breton G, Douard D, Saiag P, Huraux JM et al (2001) Clinical and virologic characterization of acyclovir-resistant varicella-zoster viruses isolated from 11 patients with acquired immunodeficiency syndrome. Clin Infect Dis 33:2061–2067
Sauerbrei A, Taut J, Zell R, Wutzler P (2011) Resistance testing of clinical varicella-zoster virus strains. Antiviral Res 90:242–247
Piret J, Boivin G (2011) Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother 55:459–472
Balfour HH Jr, Benson C, Braun J, Cassens B, Erice A, Friedman-Kien A et al (1994) Management of acyclovir-resistant herpes simplex and varicella-zoster virus infections. J Acquir Immune Defic Syndr 7:254–260
Sauerbrei A (2014) Diagnostik und antivirale Therapie von Herpes-simplex-Virus-Infektionen. Der Mikrobiol 24:151–158
Safrin S, Berger TG, Gilson I, Wolfe PR, Wofsy CB, Mills J et al (1991) Foscarnet therapy in five patients with AIDS and acyclovir-resistant varicella-zoster virus infection. Ann Intern Med 115:19–21
Morfin F, Thouvenot D (2003) Herpes simplex virus resistance to antiviral drugs. J Clin Virol 26:29–37
Safrin S, Crumpacker C, Chatis P, Davis R, Hafner R, Rush J et al (1991) A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med 325:551–555
Schubert A, Gentner E, Bohn K, Schwarz M, Mertens T, Sauerbrei A (2014) Single nucleotide polymorphisms of thymidine kinase and DNA polymerase genes in clinical herpes simplex virus type 1 isolates associated with different resistance phenotypes. Antiviral Res 107:16–22
Brunnemann AK, Bohn-Wippert K, Zell R, Henke A, Walther M, Braum O et al (2015) Drug resistance of clinical varicella-zoster virus strains confirmed by recombinant thymidine kinase expression and by targeted resistance mutagenesis of a cloned wild-type isolate. Antimicrob Agents Chemother 59:2726–2734
Oh SH, Choi EH, Shin SH, Kim YK, Chang JK, Choi KM et al (2014) Varicella and varicella vaccination in South Korea. Clin Vaccine Immunol 21:762–768
Gershon AA (1997) Live attenuated varicella vaccine. Int J Infect Dis 1:130–134
Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T, Isomura S (1974) Live vaccine used to prevent the spread of varicella in children in hospital. Lancet 2:1288–1290
Arvin A, Gershon A (2006) Control of varicella: why is a two-dose schedule necessary? Pediatr Infect Dis J 25:475–476
Luthy KE, Tiedeman ME, Beckstrand RL, Mills DA (2006) Safety of live-virus vaccines for children with immune deficiency. J Am Acad Nurse Pract 18:494–503
Damm O, Ultsch B, Horn J, Mikolajczyk RT, Greiner W, Wichmann O (2015) Systematic review of models assessing the economic value of routine varicella and herpes zoster vaccination in high-income countries. BMC Public Health 15:533
Liese JG, Cohen C, Rack A, Pirzer K, Eber S, Blum M et al (2013) The effectiveness of varicella vaccination in children in Germany: a case–control study. Pediatr Infect Dis J 32:998–1004
Gershon AA (2001) The current status of live attenuated varicella vaccine. Arch Virol Suppl 17:1–6
Chaves SS, Lopez AS, Watson TL, Civen R, Watson B, Mascola L et al (2011) Varicella in infants after implementation of the US varicella vaccination program. Pediatrics 128:1071–1077
Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX et al (2002) Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA 287:606–611
Ampofo K, Saiman L, LaRussa P, Steinberg S, Annunziato P, Gershon A (2002) Persistence of immunity to live attenuated varicella vaccine in healthy adults. Clin Infect Dis 34:774–779
Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B et al (2004) Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J 23:132–137
Marin M, Meissner HC, Seward JF (2008) Varicella prevention in the United States: a review of successes and challenges. Pediatrics 122:e744–e751
Marin M, Nguyen HQ, Keen J, Jumaan AO, Mellen PM, Hayes EB et al (2005) Importance of catch-up vaccination: experience from a varicella outbreak, Maine, 2002–2003. Pediatrics 115:900–905
Lopez AS, Cardemil C, Pabst LJ, Cullen KA, Leung J, Bialek SR et al (2014) Two-dose varicella vaccination coverage among children aged 7 years—six sentinel sites, United States, 2006–2012. MMWR Morb Mortal Wkly Rep 63:174–177
Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F (2011) Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis 52:332–340
Sauerbrei A (2014) Herpes zoster im Kindes- und Jugendalter. Monatsschr Kinderheilkd 162:1135–1144
Weinmann S, Chun C, Schmid DS, Roberts M, Vandermeer M, Riedlinger K et al (2013) Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis 208:1859–1868
Uebe B, Sauerbrei A, Burdach S, Horneff G (2002) Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child. Eur J Pediatr 161:442–444
Robert Koch-Institut (2004) Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut (Stand: Juli 2004). Epidemiol Bull 30:235–250
Streng A, Grote V, Carr D, Hagemann C, Liese JG (2013) Varicella routine vaccination and the effects on varicella epidemiology—results from the Bavarian Varicella Surveillance Project (BaVariPro), 2006–2011. BMC Infect Dis 13:303
Robert Koch-Institut (2009) Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut (Stand: Juli 2009). Epidemiol Bull 30:279–298
Robert Koch-Institut (2011) Zur Kombinationsimpfung gegen Masern, Mumps, Röteln und Varizellen (MMRV). Epidemiol Bull 38:352–353
Robert Koch-Institut (2015) Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut (Stand: August 2015). Epidemiol Bull 34:327–362
Sauerbrei A, Wutzler P (2007) Varicella-Zoster-Virus-Infektionen: Aktuelle Prophylaxe und Therapie, 2nd edn. Uni-Med, Bremen, pp 70–87
Galea SA, Sweet A, Beninger P, Steinberg SP, LaRussa PS, Gershon AA et al (2008) The safety profile of varicella vaccine: a 10-year review. J Infect Dis 197:S165–S169
Robert Koch-Institut (2005) Neuerungen in den aktuellen Impfempfehlungen der STIKO. Epidemiol Bull 31:273–276
Sauerbrei A (2011) Preventing congenital varicella syndrome with immunization. CMAJ 183:E169–E170
Arvin A (2005) Aging, immunity, and the varicella-zoster virus. N Engl J Med 352:2266–2267
Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD et al (2005) A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 352:2271–2284
Vesikari T, Hardt R, Rümke HC, Icardi G, Montero J, Thomas S et al (2013) Immunogenicity and safety of a live attenuated shingles (herpes zoster) vaccine (Zostavax®) in individuals aged ≥70 years: a randomized study of a single dose vs. two different two-dose schedules. Hum Vaccin Immunother 9:858–564
Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapelière P, Vassilev V, Ledent E et al (2012) A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein E subunit vaccine candidate in young and older adults. J Infect Dis 206:1280–1290
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author reports no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sauerbrei, A. Diagnosis, antiviral therapy, and prophylaxis of varicella-zoster virus infections. Eur J Clin Microbiol Infect Dis 35, 723–734 (2016). https://doi.org/10.1007/s10096-016-2605-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2605-0