Abstract
To diagnose invasive fungal infections, the detection of (1 → 3)-β-d-glucan in serum has shown variable specificity, depending on the targeted population. Several circumstances for false-positive results of beta-glucan tests have been identified, among which are severe bacterial infections. In this study, we measured (1 → 3)-β-d-glucan by the Fungitell test in the serum of 62 patients (one serum sample tested per patient) for whom invasive fungal infection was not suspected: 19 control subjects and 43 patients with bacteraemia. The test was interpretable for 58 sera: all 19 control subjects had negative beta-glucan test; among the 39 bacteraemic patients, we report 16 false-positive results. For the 22 patients undergoing bacteraemia due to Gram-negative bacilli, we observed 13 false-positive results (59%). Among the 17 patients with bloodstream infection involving Gram-positive cocci, three false-positive tests were recorded, but none in the eight cases of Streptococcus pneumoniae bacteraemia. Statistical analysis showed that beta-glucan levels were significantly higher in patients with Gram-negative bacilli bloodstream infection in comparison to those with bacteraemia due to Gram-positive cocci. These results were independent from other previously described causes for false-positive beta-glucan tests. These data might help physicians to interpret positive beta-glucan detection when an invasive fungal infection is suspected, especially for patients with bacterial infections.
Similar content being viewed by others
Introduction
(1 → 3)-β-d-glucan (BG) is a component of the cell wall of fungi [1]. Its presence in the bloodstream correlates with invasive fungal infection (IFI), such as Candida bloodstream infection (BSI) [2, 3], invasive aspergillosis [2, 4, 5], Pneumocystis jirovecii pneumonia [6–8] or fungaemias due to less frequent species [9].
BG is used for the diagnosis of IFI and has been included in 2008 in the criteria for probable invasive fungal disease by the European Organization for Research and Treatment of Cancer [10]. This test has been assessed in many studies [3, 11–17]. It shows good sensitivity (up to 97%) and negative predictive value (78 to 100%). The specificity and positive predictive value (PPV) vary between studies and decrease dramatically when the test is used in populations of patients at high risk for IFI. A recent study conducted in such a population reports numbers between 20 and 56% for specificity and 10 to 12% for PPV, depending on the cut-off and the number of samples (one or two) considered for a positive test [18]. Several conditions may lead to false-positive results for the BG test: haemodialysis with cellulose membranes [19], serosal exposure to gauze containing glucan [20, 21], presence of free haemoglobin in serum [16], administration of antimicrobials containing BG [22] or treatment with immunoglobulins, albumin or other blood products filtered through cellulose membranes [23].
Positive BG results were also reported for patients with bacterial infections [11, 16, 24]. Indeed, certain bacteria, such as Alcaligenes faecalis, Agrobacterium sp. or Pseudomonas aeruginosa, are known to produce BG [24, 25]. Despite the widening diagnostic use of BG tests, very few studies focused on BG detection in the serum of patients with bacteraemia. The confusing issue is that patients at risk for IFI, for whom BG testing is of great help to diagnose fungal disease, are also at risk for bacterial infections.
The aims of our study were to evaluate the reactivity of the Fungitell BG assay in bacterial BSI and to identify bacteria more frequently involved in cases of bacteraemia with high BG reactivity. This study was conducted in clinical situations where IFI was not suspected.
Patients and methods
Population and serum collection
This retrospective monocentric study was carried out at Reims University Hospital, France. It included bacteraemic patients for whom a serum sample had been taken close to the time of evidence for a bacterial BSI and was available in our biobank. Patients with proven or suspected invasive fungal disease were excluded. Over the same period, blood samples obtained from outpatients were included as controls. Only one serum sample per patient was collected for BG testing. For all of the patients, different possible causes of positive BG result were recorded: concomitant treatment with haemodialysis, recent surgery (as far as one month before serum collection), mucositis, aspect of serum, administration of antimicrobial chemotherapy or products filtered through cellulose membranes, such as immunoglobulins.
Blood cultures
Blood samples were collected in vials monitored at 37°C in a BacT/ALERT 3D incubator (bioMérieux, Lyon, France). For positive cultures, bacteria were identified by the usual biochemical systems (API strips, VITEK 2 system). Enterobacteriaceae, Staphylococcus aureus, Streptococcus pneumoniae and P. aeruginosa were considered as obligate pathogens if isolated from blood cultures (at least one positive vial) and coagulase-negative staphylococci were considered as pathogens if isolated from vials collected at different times in a consistent septic background (cutaneous portal of entry, temperature of >38.5°C, targeted antimicrobial therapy required).
BG assay
Blood samples were collected in sterile, BG-free clotting tubes. Serum was separated by centrifugation. Serum samples were stored at −80°C until testing for BG. BG levels were measured with the Fungitell test kit (Associates of Cape Cod, East Falmouth, MA, USA) in procedures as recommended by the manufacturer. The kinetic colorimetric assay performed at 37°C was read at 405 nm for 25 min. The concentration of BG in each sample was automatically calculated by using a calibration curve built with standard solutions ranging from 6.25 to 100 pg/mL. Serum assays were performed in duplicate. A test was considered to be valid when the two values obtained differed by less than 30%. According to the supplier’s specifications, a BG level of ≥80 pg/mL was considered to be positive, a BG level of <60 pg/mL was considered to be negative and values between 60 and 80 were interpreted as indeterminate.
Statistical analysis
Categorical variables were described by the frequency (and percentage) of their occurring values and continuous variables, such as the BG level, by their mean (and standard deviation). Bivariate analyses of the continuous explained variable ‘BG level’ (comparison of averages) were performed with analysis of variance (ANOVA). Multivariate analysis was carried out through linear regression, considering as input variables the significant explanatory variables according to the bivariate analyses. p-values less than 0.05 were considered to be significant. All statistical analyses were performed using SAS, version 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
A total of 62 patients were retrospectively included, with serum samples taken from May 2007 to March 2009. Patients were divided into three groups: A, control subjects (n = 19); B, patients with blood culture for Gram-negative (GN) bacilli (n = 24); C, patients with blood cultures for Gram-positive (GP) cocci (n = 19). In each of the groups B and C, two subgroups were distinguished according to the species responsible for the BSI: Escherichia coli, P. aeruginosa (in group B), S. aureus and S. pneumoniae (in group C). Group A (control subjects) included 19 immunocompetent patients tested for parasitic diseases (toxoplasmosis, helminthiases).
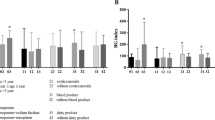
The clinical characteristics of patients with BSI (groups B and C) are shown, respectively, in Tables 1 and 2. Twenty-six patients (60%) were male. The mean age of patients was 58.1 years (± 24.2). None of the patients had received immunoglobulins, albumin or other blood products filtered through cellulose membranes. Eight patients (19%) had undergone surgery in the month before BSI. One (2%) suffered from mucositis. Four (9%) were treated with haemodialysis. Six sera (14%) were haemolysed, lipaemic or bilirubinaemic.
The time of BG testing from BSI evidence ranged from 3 days before to 7 days after. Blood collection for serum testing and for blood culture (to become positive) was concomitant for 23 patients (54%). For 8 patients (19%), serum was harvested 1 day after the collection of the positive blood culture. The time of BG testing was 2 to 3 days after BSI evidence for 4 patients (9%). It was 4 days or more for 4 patients (9%). Inversely, serum was collected before blood culture for 4 patients (9%), all with chronic bacterial infections.
BG levels
For two patients in group B (patients 23 and 24) and two patients in group C (patients 34 and 43), the BG assay was considered to be invalid, since the two values obtained on the same serum by the duplicate test differed by more than 30% (data not shown). These four sera were not tested in a second run and were excluded from the data analysis. We, therefore, analysed 58 results out of the 62 sera: 19 from group A, 22 for group B patients and 17 for patients in group C. The results of BG detection for bacteraemic patients are presented in Tables 1 and 2. The distribution of the BG test results in each group and subgroup of patients is shown in Table 3.
The results of the BG assay in group A (control subjects) were all negative, ranging from 12 to 56 pg/mL. Considering BSI (group B + group C), the BG testing was positive in 16 (41%) of 39 patients. For 13 (81%) of these 16 patients, blood cultures grew Gram-negative bacilli (eight with E. coli, one with Klebsiella oxytoca and Proteus mirabilis, four with P. aeruginosa). Among the patients in group B (with BSI due to GN bacilli), 13 samples (59%) were positive for BG, 3 (14%) were indeterminate and 6 (27%) were negative. For patients in group C (with bacteraemia due to GP cocci), 3 samples (18%) were positive for BG, 1 (6%) was indeterminate and 13 (76%) were negative. The BG levels for the eight patients of the S. pneumoniae subgroup with valid results were all negative.
The mean level of BG and standard deviation for each group (A, B, C) are, respectively: 33 ± 15 pg/mL, 127 ± 116 pg/mL and 56 ± 44 pg/mL. Concerning patients with S. pneumoniae BSI, the observed BG levels (38 ± 17 pg/mL) were identical to those measured for control subjects. Bivariate analysis highlighted that the BG level was significantly higher for patients with BSI due to GN bacilli compared to those with GP cocci bacteraemia (p = 0.02). Analysis of the factors responsible for the false-positive results of the BG test showed that the BG levels were statistically higher for patients treated with haemodialysis (p = 0.006). Regarding recent surgery, aspect of serum and the administration of antimicrobial therapy, no significant influence on the BG level was observed. These results were confirmed by multivariate analysis, including, as explanatory variables, the group of bacteria involved in BSI (GN bacilli versus GP cocci, p = 0.02) and treatment with haemodialysis (p = 0.005). Among patients not treated with haemodialysis (n = 16), the BG levels still proved to be significantly higher when BSI involved GN bacilli (p = 0.015).
Discussion
In recent years, BG assays appeared as useful non-invasive tests for the diagnosis of IFI, especially in immunocompromised patients. In this study, we did not intend to assess the diagnostic performances of the test. Our investigation focused on cases of BSI where IFI was not suspected. We observed a high proportion of false-positive results for BG measured by the Fungitell test in patients with infection due to GN bacilli. The BG levels proved to be significantly higher in patients with GN bacilli BSI compared to those with GP cocci bacteraemia, even when patients treated with haemodialysis were excluded from the statistical analysis.
It has already been proven that the cell wall of certain GN bacilli contains (1 → 3)-β-d-glucan [25, 26], especially P. aeruginosa: Mennink-Kersten et al., using the Fungitell kit, found positive BG results in sera collected from 2 out of 9 patients with P. aeruginosa bacteraemia and demonstrated high BG reactivity in the supernatant of P. aeruginosa cultures [24]. In our study, we analysed the valid results from seven patients who presented a bacteraemia due to P. aeruginosa and 15 patients who experienced a BSI due to Enterobacteriaceae (E. coli in 13 cases, Table 1). We did not observe a significant difference in BG levels between these two subgroups. Further investigations are needed in order to compare the quantity of (1 → 3)-β-d-glucan in the cell wall of distinct species of Enterobacteriaceae. It has also been suggested that the BG assay could cross-react with various bacterial carbohydrates released from the cell wall [11], such as other glucans or glucan-like polymers.
Positive results for the BG test have also been previously reported in cases of BSI due to GP cocci. Pickering et al. observed 11 (73%) positive results among 15 patients [16]. The distribution of bacterial species involved in bacteraemia may account for the apparent difference with our results (three positive BG results, i.e. 18% out of 17 patients). Indeed, Pickering et al. included nine BSI due to S. aureus (the BG test was indeterminate for one patient and negative for two others), three bacteraemias involving coagulase-negative cocci, one due to S. mitis, one with Enterococcus faecium and a single case of BSI due to S. pneumoniae (with negative BG testing). Our group of 17 BSI due to GP cocci included eight cases of bacteraemia with S. pneumoniae. The BG test was negative in all of these eight samples. Mennink-Kersten et al. observed a positive BG test in the serum of 1 out of 5 patients with S. pneumoniae bacteraemia and moderate BG reactivity in the supernatant of one S. pneumoniae culture [24]. The authors, however, limited this testing to a single bacterial strain. Further investigations are needed in order to determine whether BSI involving S. pneumoniae could account for a positive BG test. Nevertheless, we acknowledge that S. pneumoniae is, in most cases, responsible for community-acquired infections; in such clinical backgrounds, IFI are usually not suspected and the BG test is not performed.
In staphylococci bacteraemia, Pickering et al. reported 9 (75%) positive BG assays in 12 patients [16], while we observed 3 (33%) positive tests out of 9 patients. Altogether, these results suggest that staphylococci BSI are also associated to frequent positive BG results. Further investigations are needed to confirm if this association is significantly independent from other causes of false-positive BG tests.
By analogy with false-positive results for galactomannan detection [27], the administration of some antimicrobial agents has been hypothesised to be responsible for BG reactivity [22, 28, 29]. According to Marty et al., all BG tests performed in 44 solutions of intravenous antimicrobials, after dilution to the maximum plasma concentration, were found to be negative [28]. To our knowledge, the only cross-reactivity of the Fungitell assay with an antibiotic agent reported in vivo occurred with amoxicillin–clavulanate [22]. In our study, the only patient who received amoxicillin–clavulanate before their serum was collected displayed a low BG level (patient 41, Table 2). The administration of other beta-lactams (particularly piperacillin–tazobactam) did not appear as a significant cause of high BG level, which is consistent with Metan’s findings [29].
We acknowledge limits in this study. Excluding fungal infection is a major issue; it is extremely difficult owing to the lack of sensitive diagnostic tools.
Because this study is retrospective, it has not always been possible to measure the BG level in a serum sample collected at the same time as the blood culture. Thirty-five patients out of 43 had serum collected the day of the blood culture which would identify the BSI or during the three days after. The four patients for whom BG was tested before documentation of the BSI carried chronic bacterial infections. Two of them were tested positive for BG. Among the four patients with serum sample taken more than three days after the time of BSI documentation, only one had a positive BG test. If we exclude the two sera with the highest spans among all samples tested (sera collected at day 5 and day 7 after bacteraemia in patients 17 and 20, respectively), the E. coli subgroup will then show 8 (73%) false-positives out of 11 valid results.
To explain the false-positive results of the BG test in bacterial digestive infections, different hypotheses have been proposed. BSI due to Gram-negative bacteria can be associated to a digestive portal of entry [30]. If gut mucosa is damaged, the penetration into the bloodstream and transient circulation of yeast antigen could be possible [31, 32]. This mechanism has already been proposed by Ellis et al. to explain the positive results for the BG assay [33]. Similarly, lesions of the mucosa could allow dietary glucans, present in the gut lumen, to cross into the bloodstream. Yet, we observed high circulating BG levels in patients with Enterobacteriaceae bacteraemia whose assumed portal of entry was not digestive (patients 3 and 6, who were free from any other reported cause of false-positive results).
In conclusion, this study highlights the potential for false-positive results in bacterial bloodstream infections. Our findings suggest that bacteraemia involving Gram-negative bacilli could account for BG reactivity, independently of other causes for false-positive results of the BG assay. A prospective study including patients at risk for IFI undergoing BSI due to various bacteria would be warranted in order to confirm our findings.
Since systemic infections in immunocompromised patients can be caused by bacteria as well as by fungi, physicians must stay aware of this cross-reactivity reported with the Fungitell assay.
Abbreviations
- BG:
-
(1 → 3)-β-d-glucan
- BSI:
-
Bloodstream infection
- GN:
-
Gram-negative
- GP:
-
Gram-positive
- IFI:
-
Invasive fungal infection
- PPV:
-
Positive predictive value
References
Odabasi Z, Paetznick VL, Rodriguez JR, Chen E, McGinnis MR, Ostrosky-Zeichner L (2006) Differences in beta-glucan levels in culture supernatants of a variety of fungi. Med Mycol 44:267–272
Miyazaki T, Kohno S, Mitsutake K, Maesaki S, Tanaka K, Ishikawa N, Hara K (1995) Plasma (1 → 3)-β-d-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J Clin Microbiol 33:3115–3118
Obayashi T, Yoshida M, Mori T, Goto H, Yasuoka A, Iwasaki H, Teshima H, Kohno S, Horiuchi A, Ito A, Yamaguchi H, Shimada K, Kawai T (1995) Plasma (1 → 3)-β-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17–20
Marty FM, Koo S (2009) Role of (1 → 3)-β-d-glucan in the diagnosis of invasive aspergillosis. Med Mycol 47(Suppl 1):S233–S240
Pazos C, Pontón J, del Palacio A (2005) Contribution of (1 → 3)-β-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J Clin Microbiol 43:299–305
Desmet S, van Wijngaerden E, Maertens J, Verhaegen J, Verbeken E, de Munter P, Meersseman W, van Meensel B, van Eldere J, Lagrou K (2009) Serum (1,3)-β-d-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J Clin Microbiol 47:3871–3874
Pisculli ML, Sax PE (2008) Use of a serum β-glucan assay for diagnosis of HIV-related Pneumocystis jiroveci pneumonia in patients with negative microscopic examination results. Clin Infect Dis 46:1928–1930
Yasuoka A, Tachikawa N, Shimada K, Kimura S, Oka S (1996) (1 → 3) β-d-glucan as a quantitative serological marker for Pneumocystis carinii pneumonia. Clin Diagn Lab Immunol 3:197–199
Yoshida M, Obayashi T, Iwama A, Ito M, Tsunoda S, Suzuki T, Muroi K, Ohta M, Sakamoto S, Miura Y (1997) Detection of plasma (1 → 3)-β-d-glucan in patients with Fusarium, Trichosporon, Saccharomyces and Acremonium fungaemias. J Med Vet Mycol 35:371–374
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821
Digby J, Kalbfleisch J, Glenn A, Larsen A, Browder W, Williams D (2003) Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients. Clin Diagn Lab Immunol 10:882–885
Obayashi T, Negishi K, Suzuki T, Funata N (2008) Reappraisal of the serum (1 → 3)-β-d-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis 46:1864–1870
Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L (2004) β-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis 39:199–205
Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R, Tamura H, Finkelman MA, Rex JH (2005) Multicenter clinical evaluation of the (1 → 3) β-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis 41:654–659
Persat F, Ranque S, Derouin F, Michel-Nguyen A, Picot S, Sulahian A (2008) Contribution of the (1 → 3)-β-d-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol 46:1009–1013
Pickering JW, Sant HW, Bowles CAP, Roberts WL, Woods GL (2005) Evaluation of a (1 → 3)-β-d-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol 43:5957–5962
Senn L, Robinson JO, Schmidt S, Knaup M, Asahi N, Satomura S, Matsuura S, Duvoisin B, Bille J, Calandra T, Marchetti O (2008) 1,3-β-d-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin Infect Dis 46:878–885
Racil Z, Kocmanova I, Lengerova M, Weinbergerova B, Buresova L, Toskova M, Winterova J, Timilsina S, Rodriguez I, Mayer J (2010) Difficulties in using 1,3-β-d-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies—high frequency of false-positive results and their analysis. J Med Microbiol 59:1016–1022
Kanda H, Kubo K, Hamasaki K, Kanda Y, Nakao A, Kitamura T, Fujita T, Yamamoto K, Mimura T (2001) Influence of various hemodialysis membranes on the plasma (1 → 3)-β-d-glucan level. Kidney Int 60:319–323
Kanamori H, Kanemitsu K, Miyasaka T, Ameku K, Endo S, Aoyagi T, Inden K, Hatta M, Yamamoto N, Kunishima H, Yano H, Kaku K, Hirakata Y, Kaku M (2009) Measurement of (1-3)-β-d-glucan derived from different gauze types. Tohoku J Exp Med 217:117–121
Kimura Y, Nakao A, Tamura H, Tanaka S, Takagi H (1995) Clinical and experimental studies of the limulus test after digestive surgery. Surg Today 25:790–794
Mennink-Kersten MA, Warris A, Verweij PE (2006) 1,3-β-d-glucan in patients receiving intravenous amoxicillin–clavulanic acid. N Engl J Med 354:2834–2835
Usami M, Ohata A, Horiuchi T, Nagasawa K, Wakabayashi T, Tanaka S (2002) Positive (1 → 3)-β-d-glucan in blood components and release of (1 → 3)-β-d-glucan from depth-type membrane filters for blood processing. Transfusion 42:1189–1195
Mennink-Kersten MA, Ruegebrink D, Verweij PE (2008) Pseudomonas aeruginosa as a cause of 1,3-β-d-glucan assay reactivity. Clin Infect Dis 46:1930–1931
Stone BA, Clarke AE (1992) Chemistry and biology of (1 → 3)-β-d-glucans. La Trobe University Press, Melbourne, Australia
McIntosh M, Stone BA, Stanisich VA (2005) Curdlan and other bacterial (1 → 3)-β-d-glucans. Appl Microbiol Biotechnol 68:163–173
Sulahian A, Touratier S, Ribaud P (2003) False positive test for aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. N Engl J Med 349:2366–2367
Marty FM, Lowry CM, Lempitski SJ, Kubiak DW, Finkelman MA, Baden LR (2006) Reactivity of (1 → 3)-β-d-glucan assay with commonly used intravenous antimicrobials. Antimicrob Agents Chemother 50:3450–3453
Metan G, Ağkuş C, Buldu H, Koç AN (2010) The interaction between piperacillin/tazobactam and assays for Aspergillus galactomannan and 1,3-beta-d-glucan in patients without risk factors for invasive fungal infections. Infection 38:217–221
MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P (1999) Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut 45:223–228
Andrutis KA, Riggle PJ, Kumamoto CA, Tzipori S (2000) Intestinal lesions associated with disseminated candidiasis in an experimental animal model. J Clin Microbiol 38:2317–2323
Cole GT, Halawa AA, Anaissie EJ (1996) The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin Infect Dis 22(Suppl 2):S73–S88
Ellis M, Al-Ramadi B, Finkelman M, Hedstrom U, Kristensen J, Ali-Zadeh H, Klingspor L (2008) Assessment of the clinical utility of serial β-d-glucan concentrations in patients with persistent neutropenic fever. J Med Microbiol 57:287–295
Acknowledgements
The plate reader (VersaMax Plus spectrophotometer equipped with SoftMax Pro software, Molecular Devices) was placed at our disposal by the Biogenic Society (Pérols, France).
We thank P. L. Toubas for the advice and help with the writing of this article.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albert, O., Toubas, D., Strady, C. et al. Reactivity of (1 → 3)-β-d-glucan assay in bacterial bloodstream infections. Eur J Clin Microbiol Infect Dis 30, 1453–1460 (2011). https://doi.org/10.1007/s10096-011-1244-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1244-8