Abstract
The objective of this work was to evaluate the efficacy of rifampin, and its combinations with imipenem or sulbactam, in an experimental pneumonia model caused by two panresistant Acinetobacter baumannii strains (HUVR99 and HUVR113). Minimum inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) (μg/ml) of the strains were rifampin 128/>128 for both strains, imipenem 128/>256 and 256/>256 for HUVR99 and HUVR113, respectively, and sulbactam >256/>256 for both strains. In time–kill studies, at MICs, rifampin was bactericidal for both strains and sulbactam against the HUVR99 strain. Rifampin plus imipenem or sulbactam, at the MIC or mice C max, were synergistic. In vivo, against HUVR99 and HUVR113, rifampin (73% and 40%) and its combinations improved the survival with respect to the control group (20% and 0%, p < 0.05), respectively. Rifampin (87% and 46%) and its combinations improved the sterilization of blood cultures with respect to the control groups (0%, p < 0.05). In regard to the bacterial clearance from lungs, rifampin (2.57 ± 2.47 and 5.35 ± 3.03 log10 cfu/g) and its combinations with imipenem or sulbactam diminished the bacterial lung concentration with respect to the control group (10.89 ± 3.00 and 11.86 ± 0.49, p < 0.05) with both strains. In conclusion, rifampin alone or associated to imipenem or sulbactam were effective for the treatment of murine pneumonia caused by selected panresistant A. baumannii strains.
Similar content being viewed by others
Introduction
Acinetobacter baumannii is an important nosocomial pathogen [1, 2], particularly in intensive care unit settings. The treatment of these infections is challenged by the ability of this microorganism to acquire resistance to almost all antibiotics, including carbapenems [3, 4]. In these situations, alternative options are scarce, and only colistin and rifampin are active against these multidrug-resistant strains. Thus, colistin has been restored for the treatment of severe multidrug-resistant A. baumannii infections [5–7], although the mortality in infections such as ventilator-associated pneumonia remains around 40%. However, infections caused by colistin-resistant strains have been reported [8–11], which have prompted investigators to explore other alternative treatments for infections caused by these strains.
Montero et al. [12], using an experimental murine pneumonia model caused by carbapenem-resistant A. baumannii strains, found that the addition of colistin did not improve the efficacy of rifampin alone; on the contrary, imipenem plus rifampin was more efficacious than rifampin monotherapy. We also showed that rifampin was better than colistin in reducing bacterial lung concentration in an experimental murine pneumonia model caused by a carbapenem-resistant A. baumannii, and that the addition of colistin to rifampin did not improve its efficacy; however, imipenem or sulbactam plus rifampin decreased 1 log10 cfu/g of lung tissue compared with rifampin alone [13].
The aim of the present study was to evaluate the efficacy of rifampin, associated to imipenem or sulbactam, against clinical isolates of panresistant A. baumannii, including resistance to colistin, using an experimental murine pneumonia model.
Materials and methods
Bacterial strains
We used two bacteremic panresistant A. baumannii strains (HUVR99 and HUVR113) belonging to the same clone responsible for an outbreak in our hospital [8]. Isolates were identified as A. baumannii by means of Gram staining, observation of their colonial morphology and motility, use of cytochrome oxidase reaction analysis, determination of whether there was growth at 44°C, as well as by the use of a semi-automated microbiology system (MicroScan Walk-Away; Dade-Behring). Additionally, the strains were confirmed as A. baumannii by the use of amplified ribosomal DNA restriction analysis [14].
Antibacterial agents and drugs
In the in vitro assays, antibacterial agents were used as laboratory-standard powders: imipenem (Merck, Sharp & Dohme, Madrid, Spain), sulbactam (PharmaSierra, Madrid, Spain), and rifampin and colistin sulfate (Sigma-Aldrich, Madrid, Spain). For the in vivo experiments, we used imipenem and sulbactam as commercial vials from the same manufacturers as above, and rifampin from Aventis (Madrid, Spain). Sodium thiopental (5% wt/vol) was provided by B. Braun Medical S.A. (Barcelona, Spain).
Susceptibility testing and time–kill curve experiments
Minimum inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) were determined according to standard methods [15, 16]. Escherichia coli ATCC 25922 was used as a control strain. The breakpoints for resistance were those defined by the Clinical and Laboratory Standards Institute (CLSI) [15], and the French Society for Microbiology for rifampin [16].
Time–kill kinetic assays were conducted on Mueller–Hinton broth (MHB) at drug concentrations of the MIC and C max (peak drug serum concentration in C57BL/6 female healthy mice, after a single dose of 30 mg/kg intramuscularly [i.m.] for imipenem, 60 mg/kg i.m. for sulbactam, and 25 mg/kg intraperitoneally [i.p.] for rifampin). A control without antibiotics was evaluated in parallel. The initial inoculum was approximately 105 cfu/ml. Cultures were incubated at 37°C and bacterial counts were determined by plating 100 μl of ten-fold dilutions of control or test tubes, onto sheep blood agar plates (SBA), at 0, 2, 4, 8, and 24 h. Plates were incubated for 24 h at 37°C. Bactericidal activity was defined as ≥3 log10 decrease of the initial inoculum in cfu/ml [17].
In vitro synergies of rifampin plus imipenem or sulbactam against the two strains were tested with time–kill assays. Concentrations of MIC or C max of each antibacterial agent were used. Synergy was defined as ≥2 log10 decreases in bacterial concentration, in comparison with the most active drug alone [18].
The in vitro experiments were performed in duplicate. Moreover, MICs and MBCs were repeated periodically to check that the susceptibilities of both strains did not change during the in vivo experiments.
Animals
Immunocompetent C57BL/6 female mice, weighing 16 to 20 g, obtained from the University of Sevilla’s facility, were used. The in vivo studies were approved by the Ethics Committee of the Hospital Universitario Virgen del Rocío, Sevilla, Spain.
Drug pharmacokinetics
Serum drug levels were determined in groups of 21 healthy mice after the administration of a single dose of each antibacterial agent. The dosages administered were as follows: 30 mg/kg/i.m. for imipenem, 60 mg/kg/i.m. for sulbactam, and 25 mg/kg/i.p. for rifampin. After 10, 15, 30, 60, 90, 120, and 150 min, blood samples were drawn from the periorbital plexus of three anesthetized mice per timepoint. Drug concentrations were measured by a bioassay method, using Micrococcus luteus ATCC 9341, Bacillus subtilis 6633, and A. baumannii ATCC 19606 as control strains [19].
The maximum plasma concentration (C max, μg/ml), terminal half-life (t 1/2, h), and area under the concentration–time curve (AUC; μg h/ml) were calculated by a computer-assisted method (http://www.boomer.org/pkin/soft.html). The time during which the plasma concentration remained above the MIC (ΔT/MIC, h) was estimated by extrapolation from the regression line of plasma elimination.
Experimental pneumonia model in mice
A murine experimental pneumonia model was carried out using A. baumannii HUVR99 and HUVR113 strains [20]. Anesthetized animals were infected transtracheally with a final inoculum of approximately 108 cfu/ml in 50 μl of the bacterial suspension mixed 1:1 with porcine mucin (Sigma-Aldrich) diluted to 10% in saline.
Antibacterial treatment
Mice were randomly ascribed into four treatment groups for 72 h: control (non-treated), rifampin (100 mg/kg/day/i.p., q.i.d.), rifampin plus imipenem (120 mg/Kg/day/i.m., q.i.d.), and rifampin plus sulbactam (240 mg/Kg/day/i.m., q.i.d.).
Because the strains used in these experiments were resistant to antibacterial agents, the dosages were those showing efficacy in an experimental pneumonia model caused by multidrug-resistant A. baumannii [13]. These dosages obtained, for imipenem and sulbactam, plasma concentrations in the ranges of those reached in humans after standard dosing for severe infections [21]. For rifampin, the dosage obtains an AUC similar to that found in humans [22].
Therapy was initiated 4 h after the bacterial challenge, when histological features of pneumonia appeared [20]. Animals were observed for 72 h for mortality, and surviving mice were sacrificed 4 h after the last antibacterial agent dose by the administration of sodium thiopental; all of the mice were analyzed immediately after death. Thoracotomy was carried out and 100 μl of blood, collected by cardiac puncture, was cultured in tryptic soy broth (TSB) for 24 h at 37°C and then 100 μl was plated on SBA plates and incubated for another 24 h at 37°C; the results were expressed as positive or negative. Heart and lungs were extracted in block and lungs were separated onto a sterile Petri plate, weighed, and processed for quantitative culture after being homogenized (Stomacher 80 Tekmar Co., Cincinnati, Ohio, USA) in 2 ml of sterile saline solution. After 10-fold dilution, aliquots of 100 μl were plated onto SBA plates for 24 h at 37°C. If cultures were negative, lungs were considered to be sterile when no growth was observed after plating the whole residue of the homogenized tissue onto SBA plates. The results were expressed as means ± standard deviations of the log10 cfu/g of lung tissue.
Lung samples were processed for pathological studies in five mice from each control group. Lungs were fixed with 10% formaldehyde and embedded in paraffin and cut into 4-μm-thick sections. The slices included all of the pulmonary lobes to be studied by optical microscopy. They were processed according to standard methods for hematoxylin-eosin, periodic acid-Schiff, Gram, Masson’s trichromic, and silver reticulin stains.
To confirm that the antibacterial treatments were not toxic to the animals, groups of five randomly non-infected mice were each given the antibiotics for 72 h.
Statistical analysis
Therapeutic efficacy in each experiment was assessed comparing the lung bacterial counts and the percentages of bacteremia and mortality among the groups. Numbers of surviving animals and negative blood cultures were evaluated with Fisher’s exact test. The cfu/g of lung tissue was analyzed using the homogeneity of variance (ANOVA) and posthoc tests (Tukey–Kramer and Dunnett test). The SPSS 14.0 statistical package (SPSS Inc., Chicago, IL, USA) was used. A p-value of 0.05 was considered to be significant.
Results
In vitro studies
The MICs and MBCs of imipenem, sulbactam, rifampin, and colistin for A. baumannii HUVR99 and HUVR113 are shown in Table 1.
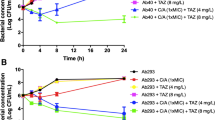
For the time–kill experiments (Figs. 1 and 2), MICs >256 μg/ml were fixed at 256 μg/ml. Against HUVR99, sulbactam was bactericidal at MIC at 8 h and at a concentration of C max at 24 h, and rifampin at MIC at 4 and 8 h; against HUVR113, only rifampin at MIC was bactericidal at 4 h. For both strains, synergy was found with rifampin plus imipenem at both concentrations, MIC and C max. Rifampin plus sulbactam was synergistic at MIC for HUVR99 and at MIC and C max for HUVR113.
Time–kill assays of rifampin, imipenem, sulbactam, and the combinations of rifampin plus imipenem or sulbactam against Acinetobacter baumannii HUVR99 at the minimum inhibitory concentration (MIC) and serum mice C max. Control, filled diamonds; rifampin (MIC), filled triangles; rifampin (C max), open squares; imipenem (MIC), filled squares; imipenem (C max), filled circles; sulbactam (MIC), open inverted triangles; sulbactam (C max), open diamonds; rifampin plus imipenem (MIC), open circles; rifampin plus imipenem (C max), inverted filled triangles; rifampin plus sulbactam (MIC), open triangles; rifampin plus sulbactam (C max), black crosses
Time–kill assays of rifampin, imipenem, sulbactam, and the combinations of rifampin plus imipenem or sulbactam against A. baumannii HUVR113 at the MIC and serum mice C max. Control, filled diamonds; rifampin (MIC), filled triangles; rifampin (C max), open squares; imipenem (MIC), filled squares; imipenem (C max), filled circles; sulbactam (MIC), open inverted triangles; sulbactam (C max), open diamonds; rifampin plus imipenem (MIC), open circles; rifampin plus imipenem (C max), inverted filled triangles; rifampin plus sulbactam (MIC), open triangles; rifampin plus sulbactam (C max), black crosses
Pharmacokinetic studies
The pharmacokinetic and pharmacodynamic parameters of each antibacterial agent are shown in Table 2. The ΔT/MIC for all of the antibacterial agents was 0 h for both strains.
Pathological studies
The mice groups inoculated with strains HUVR99 and HUVR113 showed alterations compatible with acute pneumonia. They presented acute inflammation, characterized by diffuse and/or focal affectation of all lobes, with a mild to severe inflammatory infiltration of polymorphonuclear cells (PMN), sometimes forming segmentary abscesses, and with mild to moderate infiltration of alveolar macrophages. Gram-negative bacterial colonies and alveolar hemorrhagic areas were also observed.
Therapeutic efficacy in experimental pneumonia
The in vivo efficacy of the antibacterial agents against both A. baumannii strains is shown in Table 3. Survival rates in the control groups were 20% and 0% for strains HUVR99 and HUVR113, respectively. For both strains, all of the treatments improved the survival rates compared with their controls (p < 0.05), with no difference among the different treatments. In the sterilization of blood cultures, for the strain HUVR99, all the treatments were efficacious (p < 0.05) compared with the control group, with no difference among the antibacterial agents. For the strain HUVR113, only rifampin and its combination with sulbactam increased the number of sterile blood cultures (46% and 93.3% vs. 0%, respectively); moreover, rifampin plus sulbactam was better than the other treatments (93.3% vs. 46% and 21%, p < 0.05). Against both A. baumannii strains, HUVR99 and HUVR113, all treatment groups decreased the lung bacterial counts when compared with the controls (p < 0.05). While for strain HUVR99 there was no differences in lung clearance among the treatment groups, against the HUVR113 strain, rifampin plus sulbactam produced higher lung clearance than that obtained with rifampin alone or when combined with imipenem (2.33 ± 1.43 vs. 5.35 ± 3.03 and 6.80 ± 2.19, respectively [p < 0.05]).
No toxicity was observed in the non-infected mice with the different therapies given during the 72-h period.
Discussion
The present study shows that rifampin and its combinations with imipenem or sulbactam are efficacious in the experimental pneumonia model caused by two A. baumannii panresistant strains, in terms of diminished mortality and clearance of bacteria from blood and lung tissue. In general, rifampin alone showed the same efficacy as its combinations, but rifampin plus sulbactam was better than the monotherapy with rifampin in the clearance of bacteria from blood and lung against one of the strains. These results are in accordance with the synergistic effect found in the time–kill studies with this combination.
This therapeutic efficacy of rifampin agrees with the results found in several experimental murine pneumonia models, using multiresistant A. baumannii strains. Wolff et al. in a pneumonia model in neutropenic mice caused by using two A. baumannii strains with different susceptibilities to rifampin [23], 8 and 4 μg/ml, respectively, observed improved survival and a decrease in bacterial lung concentration with rifampin. Montero et al., in an immunocompetent murine pneumonia model [12], reported similar results with three A. baumannii strains with MICs of rifampin of 8 μg/ml. We also observed [13], in two experimental animal models of pneumonia in immunocompetent mice and meningitis in rabbits, using an imipenem-resistant A. baumannii strain with an MIC of rifampin of 4 μg/ml, the therapeutic efficacy of this antibacterial agent alone and in combination, also in terms of improved survival and a decrease of bacterial lung concentration.
Saballs et al. treated ten patients with A. baumannii infections with rifampin plus imipenem [24], curing eight cases in spite of the development of resistance to rifampin in seven cases caused by strains with MICs of imipenem of >256, 128, and 64 μg/ml in five, one, and one cases, respectively.
The mechanisms by which rifampin was active in the experimental pneumonia caused by highly rifampin-resistant A. baumannii remains to be elucidated. The first may be the synergistic in vitro effect of the two combinations, rifampin plus imipenem or sulbactam, avoiding the regrowth of A. baumannii at 24 h at C max, except in the case of imipenem in the HUVR99 strain, suggesting the usefulness of these combinations for the treatment of infections by panresistant A. baumannii. Other possibilities deserved posterior studies.
Some antibacterial agents, such as macrolides (clarithromycin or erythromycin), azalides (azithromycin), and ketolides (telithromycin), present higher levels at the infection site than that found in plasma [25], due to the accumulation in PMN or epithelial lining fluid. Fluoroquinolones, such as ciprofloxacin, also present much higher concentrations in the lung tissue than in plasma [26]. With respect to rifampin, previous studies have shown accumulation inside PMN cells [27] or in alveolar macrophages, reaching 16.26 times the plasmatic concentration (251.8 and 15.1 μg/ml, respectively) [28]. These accumulations could explain the effectiveness of rifampin in the treatment of pulmonary infections. In this way, the effectiveness of rifampin in the experimental pneumonia model by panresistant A. baumannii strains may be due to an accumulation of the drug in the alveolar macrophages, sufficient to eradicate the infection caused by this pathogen, or its transport to the infection site by PMN cells.
The conditions under which a drug interacts with a pathogen in vivo are different to those under which the studies are carried out in vitro. Thus, the pH of the lung—the infection site in the experimental model of A. baumannii of the present study—has an acid pH as opposed to the neutral pH of the medium in which the MIC is determined. This lower pH alters the effectiveness of the antibacterial agents [29]. Therefore, one hypothesis is that the acidity of the pH of the lung could increase the susceptibility of A. baumannii to rifampin.
Rifampin has two major active metabolites; 25-O-deacetylrifampicin produced by deacetylation at the hepatic level and 3-formylrifamycin SV produced by hydrolysis at acid pH. Both are detectable in serum and urine [30, 31]. It may be that the A. baumannii strains used in this study display a greater susceptibility to these metabolites than to rifampin. This could explain why rifampin is more active against A. baumannii in vivo than in vitro, since, in this last situation, the metabolites derivates from rifampin would not be generated.
In summary, the results of the present study suggest that rifampin and its combinations with imipenem or sulbactam may be efficacious in the treatment of pulmonary infections caused by panresistant strains of A. baumannii. However, because of the high MIC of rifampin against the A. baumannii strains used in this study, the mechanisms of this in vivo experimental efficacy of rifampin must be elucidated before the evaluation of this therapy in humans.
References
Rodríguez-Baño J, Cisneros JM, Fernández-Cuenca F; Grupo de Estudio de Infección Hospitalaria (GEIH) et al (2004) Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol 25:819–824
Rodríguez-Baño J, García L, Ramírez E et al (2009) Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive “bundle” approach. Am J Infect Control 37:715–722
Michalopoulos A, Falagas ME (2010) Treatment of Acinetobacter infections. Expert Opin Pharmacother 11:779–788
McGowan JE Jr (2006) Resistance in nonfermenting gram-negative bacteria: multidrug resistance to the maximum. Am J Med 119(6 Suppl 1):S29–S36; discussion S62–S70
Falagas ME, Kasiakou SK, Tsiodras S et al (2006) The use of intravenous and aerosolized polymyxins for the treatment of infections in critically ill patients: a review of the recent literature. Clin Med Res 4:138–146
Garnacho-Montero J, Ortiz-Leyba C, Jiménez-Jiménez FJ et al (2003) Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis 36:1111–1118
De Pascale G, Pompucci A, Maviglia R et al (2010) Successful treatment of multidrug-resistant Acinetobacter baumannii ventriculitis with intrathecal and intravenous colistin. Minerva Anestesiol 76:957–960
Valencia R, Arroyo LA, Conde M et al (2009) Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol 30:257–263
Cisneros-Herreros JM, Garnacho-Montero J, Pachón-Ibáñez ME (2005) Nosocomial pneumonia due to Acinetobacter baumannii. Enferm Infecc Microbiol Clín 23(Suppl 3):46–51
Li J, Nation RL, Owen RJ et al (2007) Antibiograms of multidrug-resistant clinical Acinetobacter baumannii: promising therapeutic options for treatment of infection with colistin-resistant strains. Clin Infect Dis 45:594–598
Ko KS, Suh JY, Kwon KT et al (2007) High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother 60:1163–1167
Montero A, Ariza J, Corbella X et al (2004) Antibiotic combinations for serious infections caused by carbapenem-resistant Acinetobacter baumannii in a mouse pneumonia model. J Antimicrob Chemother 54:1085–1091
Pachón-Ibáñez ME, Docobo-Pérez F, López-Rojas R et al (2010) Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 54:1165–1172
Vaneechoutte M, Dijkshoorn L, Tjernberg I et al (1995) Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J Clin Microbiol 33:11–15
Clinical and Laboratory Standards Institute (CLSI) (2010) Performance standards for antimicrobial susceptibility testing; Twentieth informational supplement. Document M100-S20. CLSI, Wayne, PA
Société Française de Microbiologie (SFM) (2010) Comite de l’antibiogramme de la Société Française de Microbiologie. SFM, Paris, France. Home page at: http://www.sfm-microbiologie.org/pages/?all=accueil, accessed August 11, 2010
National Committee for Clinical Laboratory Standards (NCCLS) (1999) Methods for determining bactericidal activity of antimicrobial agents: approved standard M26-A. NCCLS, Wayne, PA
Pillai SK, Moellering RC Jr, Eliopoulos GM (2005) Antimicrobial combinations. In: Lorian V (ed) Antibiotics in laboratory medicine, 5th edn. Lippincott Williams & Wilkins, Philadelphia, PA, pp 365–440
Klein RD, Edberg SC, Lorian V (2005) Applications, significance of, and methods for the measurement of antimicrobial concentrations in human body fluids. In: Lorian V (ed) Antibiotics in laboratory medicine, 5th edn. Lippincott Williams & Wilkins, Philadelphia, PA, pp 290–364
Rodríguez-Hernández MJ, Pachón J, Pichardo C et al (2000) Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J Antimicrob Chemother 45:493–501
Amsden GW (2010) Table of antimicrobial agent pharmacology. In: Mandell GL, Bennett JE, Dolin R (eds) Principles and practice of infectious diseases, 7th edn. Churchill Livingstone Elsevier, Philadelphia, PA, pp 705–761
Ruslami R, Nijland HM, Alisjahbana B et al (2007) Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother 51:2546–2551
Wolff M, Joly-Guillou ML, Farinotti R et al (1999) In vivo efficacies of combinations of beta-lactams, beta-lactamase inhibitors, and rifampin against Acinetobacter baumannii in a mouse pneumonia model. Antimicrob Agents Chemother 43:1406–1411
Saballs M, Pujol M, Tubau F et al (2006) Rifampicin/imipenem combination in the treatment of carbapenem-resistant Acinetobacter baumannii infections. J Antimicrob Chemother 58:697–700
Drusano GL (2005) Infection site concentrations: their therapeutic importance and the macrolide and macrolide-like class of antibiotics. Pharmacotherapy 25:150S–158S
Bamberger DM, Foxworth JW, Bridwell DL, Shain CS, Gerding DN (2005) Extravascular antimicrobial distribution and the respective blood and urine concentrations in humans. In: Lorian V (ed) Antibiotics in laboratory medicine, 5th edn. Lippincott Williams & Wilkins, Philadelphia, PA, pp 719–814
Prokesch RC, Hand WL (1982) Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother 21:373–380
Ziglam HM, Baldwin DR, Daniels I et al (2002) Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother 50:1011–1015
Barcia-Macay M, Seral C, Mingeot-Leclercq MP et al (2006) Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother 50:841–851
Inderlied CB, Nash KA (2005) Antimycobacterial agents: in vitro susceptibility testing, spectra of activity, mechanisms of action and resistance, and assays for activity in biologic fluids. In: Lorian V (ed) Antibiotics in laboratory medicine. Lippincott Williams & Wilkins, Philadelphia, PA, pp 155–225
Weber A, Opheim KE, Smith AL et al (1983) High-pressure liquid chromatographic quantitation of rifampin and its two major metabolites in urine and serum. Rev Infect Dis 5:S433–S439
Acknowledgments
This work was supported by research grants from the Consejería de Salud de la Junta de Andalucía (13/02) and the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III—co-financed by the European Development Regional Fund “A way to achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008/0000).
Author information
Authors and Affiliations
Corresponding author
Additional information
María Eugenia Pachón-Ibáñez and Fernando Docobo-Pérez contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Pachón-Ibáñez, M.E., Docobo-Pérez, F., Jiménez-Mejias, M.E. et al. Efficacy of rifampin, in monotherapy and in combinations, in an experimental murine pneumonia model caused by panresistant Acinetobacter baumannii strains. Eur J Clin Microbiol Infect Dis 30, 895–901 (2011). https://doi.org/10.1007/s10096-011-1173-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1173-6