Abstract
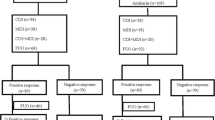
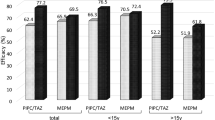
Patients with fever and granulocytopenia are at risk of developing severe infection. We performed a prospective, randomized trial to evaluate the efficacy of low-dose cefepime plus amikacin (C-A) compared to low-dose piperacillin/tazobactam plus amikacin (PT-A). Patients received cefepime (2 g/12 h) plus amikacin (15 mg/kg/day) or piperacillin/tazobactam (4 g/500 mg/8 h) plus amikacin. A total of 317 episodes of febrile granulocytopenia in 190 patients were studied (152 in the C-A group, 165 in the PT-A group). A microbiologically documented infection was present in 53 (35%) episodes in the C-A group and 41 (25%) episodes in the PT-A group (p = ns); a clinically documented infection was observed in 39 (26%) and 47 (28%) episodes, respectively. Toxicity was observed in 6 (4%) episodes in the C-A group and in 5 (3%) episodes in the PT-A group. The antibiotic success rate (no change or addition of antibiotics) was recorded in 89 (59%) and 105 (64%) cases, respectively (p = ns). Mortality related to infection was similar in each arm (3.9% vs. 3.6%). Combination therapy of low-dose β-lactam with an aminoglycoside achieves very good response rates and low rates of toxicity. It might be an attractive option in an environment of increasing resistance among gram-negative bacteria.
Similar content being viewed by others
References
Bodey GP, Buckley M, Sathe YS, Freireich EJ (1966) Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64:328–340
Wade JC, Schimpff SC, Newman KA, Fortner CL, Standiford HC, Wiernik PH (1981) Piperacillin or ticarcillin plus amikacin. A double-blind prospective comparison of empiric antibiotic therapy for febrile granulocytopenic cancer patients. Am J Med 71:983–990
EORTC (1987) Ceftazidime combined with a short or long course of amikacin for empirical therapy of gram-negative bacteremia in cancer patients with granulocytopenia. N Engl J Med 317:1692–1698
Cordonnier C, Herbrecht R, Pico JL, Gardembas M, Delmer A, Delain M et al (1997) Cefepime/amikacin versus ceftazidime/amikacin as empirical therapy for febrile episodes in neutropenic patients: a comparative study. Clin Infect Dis 24:41–51
Leyland MJ, Bayston KF, Cohen J, Warren R, Newland AC, Bint AJ et al (1992) A comparative study of imipenem versus piperacillin plus gentamicin in the initial management of febrile neutropenic patients with haematological malignancies. J Antimicrob Chemother 30:843–854
Eggimann P, Glauser MP, Aoun M, Meunier F, Calandra T (1993) Cefepime monotherapy for the empirical treatment of fever in granulocytopenic cancer patients. J Antimicrob Chemother 32(Suppl B):151–163
Freifeld AG, Walsh T, Marshall D, Gress J, Steinberg SM, Hathorn J et al (1995) Monotherapy for fever and neutropenia in cancer patients: a randomized comparison of ceftazidime versus imipenem. J Clin Oncol 13:165–176
Cometta A, Calandra T, Gaya H, Zinner SH, de Bock R, del Favero A et al (1996) Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. Antimicrob Agents Chemother 40:1108–1115
Biron P, Fuhrmann C, Cure H, Viens P, Lefebvre D, Thyss A et al (1998) Cefepime versus imipenem–cilastatin as empirical monotherapy in 400 febrile patients with short duration neutropenia. J Antimicrob Chemother 42:511–518
Bratu S, Landman D, Alam M, Tolentino E, Quale J (2005) Detection of KPC carbapenem-hydrolyzing enzymes in Enterobacter spp. from Brooklyn, New York. Antimicrob Agents Chemother 49(2):776–778
Woodford N, Tierno PM Jr, Young K, Tysall L, Palepou MF, Ward E et al (2004) Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York Medical Center. Antimicrob Agents Chemother 48(12):4793–4799
Navon-Venezia S, Chmelnitsky I, Leavitt A, Schwaber MJ, Schwartz D et al (2006) Plasmid-mediated imipenem-hydrolyzing enzyme KPC-2 among multiple carbapenem-resistant Escherichia coli clones in Israel. Antimicrob Agents Chemother 50(9):3098–3101
Bratu S, Landman D, Haag R, Recco R, Eramo A, Alam M et al (2005) Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med 165(12):1430–1435
Hossain A, Ferraro MJ, Pino RM, Dew RB 3rd, Moland ES, Lockhart TJ et al (2004) Plasmid-mediated carbapenem-hydrolyzing enzyme KPC-2 in an Enterobacter sp. Antimicrob Agents Chemother 48(11):4438–4440
Bratu S, Brooks S, Burney S, Kochar S, Gupta J, Landman D et al (2007) Detection and spread of Escherichia coli possessing the plasmid-borne carbapenemase KPC-2 in Brooklyn, New York. Clin Infect Dis 44(7):972–975
Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A (2007) Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis 45:753–760
Figuera A, Rivero N, Pajuelo F, Font P, Leyra F, de la Cámara R et al (2001) Comparative study of piperacillin/tazobactam versus imipenem/cilastatin in febrile neutropenia (1994–1996). Med Clin (Barc) 116:610–611
Sanz MA, López J, Lahuerta JJ, Rovira M, Batlle M, Pérez C et al (2002) Cefepime plus amikacin versus piperacillin–tazobactam plus amikacin for initial antibiotic therapy in haematology patients with febrile neutropenia: results of an open, randomized, multicentre trial. J Antimicrob Chemother 50(1):79–88
Montalar J, Segura A, Bosch C, Galán A, Juan O, Molins C et al (2002) Cefepime monotherapy as an empirical initial treatment of patients with febrile neutropenia. Med Oncol 19(3):161–166
Raad II, Escalante C, Hachem RY, Hanna HA, Husni R, Afif C et al (2003) Treatment of febrile neutropenic patients with cancer who require hospitalization: a prospective randomized study comparing imipenem and cefepime. Cancer 98:1039–1047
Del Favero A, Menichetti F, Martino P, Bucaneve G, Micozzi A, Gentile G et al (2001) A multicenter, double-blind, placebo-controlled trial comparing piperacillin–tazobactam with and without amikacin as empiric therapy for febrile neutropenia. Clin Infect Dis 33:1295–1301
Tamura K, Imajo K, Akiyama N, Suzuki K, Urabe A, Ohyashiki K et al (2004) Randomized trial of cefepime monotherapy or cefepime in combination with amikacin as empirical therapy for febrile neutropenia. Clin Infect Dis 39:S15–S24
Schentag JJ, Strenkoski-Nix LC, Nix DE, Forrest A (1998) Pharmacodynamic interactions of antibiotics alone and in combination. Clin Infect Dis 27:40–46
Feld R (1998) Criteria for response in patients in clinical trials of empiric antibiotic regimens for febrile neutropenia. Is there agreement? Support Care Cancer 6:444–448
World Health Organization (1979) WHO handbook for reporting results of cancer treatment. World Health Organization, Geneva, pp 16–21
Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T et al (2002) 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34:730–751
Jándula BM, Martino R, Gurgi M, Manteiga R, Sierra J (2001) Treatment of febrile neutropenia with cefepime monotherapy. Chemotherapy 47(3):226–231
Cherif H, Björkholm M, Engervall P, Johansson P, Ljungman P, Hast R et al (2004) A prospective, randomized study comparing cefepime and imipenem–cilastatin in the empirical treatment of febrile neutropenia in patients treated for haematological malignancies. Scand J Infect Dis 36:593–600
Cornely OA, Bethe U, Seifert H, Breuer K, Schütt-Gerowitt H, Salzberger B et al (2002) A randomized monocentric trial in febrile neutropenic patients: ceftriaxone and gentamicin vs cefepime and gentamicin. Ann Hematol 81(1):37–43
Bow EJ, Rotstein C, Noskin GA, Laverdière M, Schwarer AP, Segal BH et al (2006) A randomized, open-label, multicenter comparative study of the efficacy and safety of piperacillin–tazobactam and cefepime for the empirical treatment of febrile neutropenic episodes in patients with hematologic malignancies. Clin Infect Dis 43:447–459
Gorschlüter M, Hahn C, Fixson A, Mey U, Ziske C, Molitor E et al (2003) Piperacillin–tazobactam is more effective than ceftriaxone plus gentamicin in febrile neutropenic patients with hematological malignancies: a randomized comparison. Support Care Cancer 11(6):362–370
Cappelletty DM (1999) Evaluation of several dosing regimens of cefepime, with various simulations of renal function, against clinical isolates of Pseudomonas aeruginosa in a pharmacodynamic infection model. Antimicrob Agents Chemother 43(1):129–133
Paul M, Yahav D, Fraser A, Leibovici L (2006) Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 57:176–189
Yahav D, Paul M, Fraser A, Sarid N, Leibovici L (2007) Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 7(5):338–348
Gomez L, Estrada C, Gomez I, Marquez M, Dalmau D, Estany C et al. Cefepime plus amikacin versus piperacillin–tazobactam plus amikacin in the treatment of patients with fever and granulocytopenia. In: Proceedings of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 16-19 December 2001. Abstract no. L-771
Nguyen TD, Williams B, Trang E (2009) Cefepime therapy and all-cause mortality. Clin Infect Dis 48:902–904
Cefepime (marketed as Maxipime) Update of Ongoing Safety Review. FDA alert (06/17/2009)
Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R et al (2000) The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol 18(16):3038–3051
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez, L., Estrada, C., Gómez, I. et al. Low-dose β-lactam plus amikacin in febrile neutropenia: cefepime vs. piperacillin/tazobactam, a randomized trial. Eur J Clin Microbiol Infect Dis 29, 417–427 (2010). https://doi.org/10.1007/s10096-010-0879-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-010-0879-1