Abstract
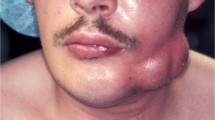
The aim of this study targeted the evaluation of the in vivo effect of moxifloxacin in the treatment of patients with severe odontogenic abscesses. This was a prospective, two-armed, randomised, unblinded, monocentric pilot study, which enrolled 21 hospitalized patients with severe odontogenic abscesses. After extraoral incision, patients were either treated with moxifloxacin 400 mg i.v. once daily or amoxicillin/clavulanic acid 2.2 g i.v. three times daily. Primary clinical endpoint was the time until clinical remission, represented by simultaneous assertion of the following criteria: body temperature <38.5°C, no pain at palpation, and mouth opening similar or better than preoperatively. White blood cell count, C-reactive protein, pain, health related quality of life (HR-QoL) and length of hospital stay were recorded as secondary outcome criteria. The mean duration until reaching the primary end point was 6.6 (range, 4.3–8.8) days in the moxifloxacin group and 6.0 (range, 3.8–8.2) days in the amoxicillin/clavulanic acid group. Median days of in-house treatment ranged between five and six days for both groups. HR-QoL was highly impaired in both groups preoperatively and reached near normal on days three and four in both samples. In this pilot investigation, moxifloxacin showed promising results as compared to amoxicillin/clavulanic acid. Therefore, a larger prospective clinical trial using moxifloxacin in severe odontogenic abscesses appears encouraging. We suggest a combination of body temperature, palpatory pain, and subjective pain as a parameter for successful intervention; however, both findings need prospective validation by means of a phase III evaluation.






Similar content being viewed by others
References
Peterson LJ (1993) Contemporary management of deep infections of the head and neck. J Oral Maxillofac Surg 51:226–231
Isenberg JS, Smith K, Tu Q (1997) Necrotizing fasciitis of the periorbita and the forehead. J Oral Maxillofac Surg 55:521–523 doi:10.1016/S0278–2391(97)90707–2
Mischkowski RA, Hidding J, Gruber G, Klesper B, Fangmann R (1997) Risikofaktoren und Management von Abszessen im MKG-Bereich - Eine retrospektive Studie von über 1000 Fällen. Dtsch Zahnarztl Z 52:697–700
Garatea-Crelogo J, Gay-Escoda C (1991) Mediastinitis from odontogenic infection. Report of three cases and review of literature. Int J Oral Maxillofac Surg 20:65–68 doi:10.1016/S0901–5027(05)80707–6
Li X, Tronstad L, Olsen I (1999) Brain abscesses caused by oral infection. Endod Dent Traumatol 15(3):95–101 doi:10.1111/j.1600–9657.1999.tb00763.x
Al-Nawas B, Maeurer M (2007) Severe versus local odontogenic bacterial infections: comparison of microbial isolates. Eur Surg Res 40(2):220–224 doi:10.1159/000110864
Lewis MA, Parkhurst CL, Douglas CW, Martin MV, Absi EG, Bishop PA et al (1995) Prevalence of penicillin resistant bacteria in acute suppurative oral infection. J Antimicrob Chemother 35(6):785–791 doi:10.1093/jac/35.6.785
Schaumann R, Ackermann G, Pless B, Claros MC, Goldstein EJ, Rodloff AC (2000) In vitro activities of fourteen antimicrobial agents against obligately anaerobic bacteria. Int J Antimicrob Agents 16(3):225–232 doi:10.1016/S0924–8579(00)00186–2
Ackermann G, Schaumann R, Pless B, Claros MC, Goldstein EJ, Rodloff AC (2000) Comparative activity of moxifloxacin in vitro against obligately anaerobic bacteria. Eur J Clin Microbiol Infect Dis 19(3):228–232 doi:10.1007/s100960050465
Buff S, Al-Nawas B, Hohlfelder M, Schulze R, Grötz KA, Maeurer M et al (2001) Anaerobier bei submukösen und Logenabszessen - therapierelevante mikrobiologische Unterschiede. Dtsch Zahnarztl Z 56(5):335–338
Vogel F, Scholz H, Al-Nawas B, Elies W, Kresken M, Lode H et al (2002) Rational use of oral antibiotics. Findings of an expert commission of the Paul Ehrlich Society for Chemotherapy. Med Monatsschr Pharm 25(6):193–204
Fille M, Mango M, Lechner M, Schaumann R (2006) Bacteroides fragilis group: trends in resistance. Curr Microbiol 52(2):153–157 doi:10.1007/s00284–005–0249-x
Wagner J, Hahn H (1996) In-vitro-Empfindlichkeit von aus Patientenmaterial isolierten anaeroben Bakterien. Chemother J 5:143–149
Al-Nawas B (2001) Infektionen im Zahn-, Mund- und Kieferbereich: Was hat sich in den letzten 25 Jahren geändert? ZMK 12:761–763
Sobottka I, Cachovan G, Stürenburg E, Ahlers MO, Laufs R, Platzer U et al (2002) In vitro activity of moxifloxacin against bacteria isolated from odontogenic abscesses. 12th European congress of clinical microbiology and infectious diseases, Milan, Italy
Firsov AA, Lubenko IY, Vostrov SN, Kononenko OV, Zinner SH, Portnoy YA (2000) Comparative pharmacodynamics of moxifloxacin and levofloxacin in an in vitro dynamic model: prediction of the equivalent AUC/MIC breakpoints and equiefficient doses. J Antimicrob Chemother 46(5):725–732 doi:10.1093/jac/46.5.725
Goldstein EJ, Citron DM, Merriam CV, Warren Y, Tyrrell K (2000) Comparative in vitro activities of GAR-936 against aerobic and anaerobic animal and human bite wound pathogens. Antimicrob Agents Chemother 44(10):2747–2751 doi:10.1128/AAC.44.10.2747–2751.2000
Eckhardt C, Fickweiler K, Schaumann R, Ackermann G, Rodloff AC (2003) Therapeutic efficacy of moxifloxacin in a murine model of severe systemic mixed infection with Escherichia coli and Bacteroides fragilis. Anaerobe 9(4):157–160 doi:10.1016/S1075–9964(03)00086–6
Kleinkauf N, Ackermann G, Schaumann R, Rodloff AC (2001) Comparative in vitro activities of gemifloxacin, other quinolones, and nonquinolone antimicrobials against obligately anaerobic bacteria. Antimicrob Agents Chemother 45(6):1896–1899 doi:10.1128/AAC.45.6.1896–1899.2001
Behra-Miellet J, Dubreuil L, Jumas-Bilak E (2002) Antianaerobic activity of moxifloxacin compared with that of ofloxacin, ciprofloxacin, clindamycin, metronidazole and beta-lactams. Int J Antimicrob Agents 20(5):366–374 doi:10.1016/S0924–8579(02)00209–1
Hoeffken G, Meyer HP, Winter J, Verhoef L (2001) The efficacy and safety of two oral moxifloxacin regimens compared to oral clarithromycin in the treatment of community-acquired pneumonia. Respir Med 95(7):553–564 doi:10.1053/rmed.2001.1113
Schaumann R, Blatz R, Beer J, Ackermann G, Rodloff AC (2004) Effect of moxifloxacin versus imipenem/cilastatin treatment on the mortality of mice infected intravenously with different strains of Bacteroides fragilis and Escherichia coli. J Antimicrob Chemother 53(2):318–324 doi:10.1093/jac/dkh089
Lode H, Kubin R, Reiter C (2002) Safety update of oral moxifloxacin: a review of worldwide post-marketing surveillance. 12th European congress of clinical microbiology and infectious diseases, Milan, Italy
Stass H, Dalhoff A, Kubitza D, Schuhly U (1998) Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother 42(8):2060–2065
Gehanno P, Darantiere S, Dubreuil C, Chobaut JC, Bobin S, Pages JC et al (2002) A prospective, multicentre study of moxifloxacin concentrations in the sinus mucosa tissue of patients undergoing elective surgery of the sinus. J Antimicrob Chemother 49(5):821–826 doi:10.1093/jac/dkf014
Goldstein EJ, Citron DM, Merriam CV, Warren Y, Tyrrel KL, Fernandez H (2003) In vitro activities of telithromycin and 10 oral agents against aerobic and anaerobic pathogens isolated from antral puncture specimens from patients with sinusitis. Antimicrob Agents Chemother 47(6):1963–1967 doi:10.1128/AAC.47.6.1963–1967.2003
Milazzo I, Blandino G, Musumeci R, Nicoletti G, Lo Bue AM, Speciale A (2002) Antibacterial activity of moxifloxacin against periodontal anaerobic pathogens involved in systemic infections. Int J Antimicrob Agents 20:451–456 doi:10.1016/S0924–8579(02)00190–5
Al-Nawas B, Grötz KA, Brahm R, Maeurer M, Wagner W (2000) Infektionen im Mund-, Kiefer- und Gesichtsbereich: Was hat sich in den letzten 25 Jahren geändert? Dtsch Zahnarztl Z 55:765–769
Karbach J, Callaway A, Willershausen B, Wagner W, Al-Nawas B (2007) Antibiotic resistance testing of the total implant-associated microflora and its pure isolates. Eur J Med Res 12:120–128
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 354(9193):1896–1900 doi:10.1016/S0140–6736(99)04149–5
Ahlers MO, Freesmeyer W, Göz G, Jakstat H, Koeck B, Meyer G et al (2003) Stellungnahme der DGZMK: Klinische Funktionsanalyse. DZZ 58:383–384
Greiner W, Weijnen T, Nieuwenhuizen M, Oppe S, Badia X, Busschbach J et al (2003) A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ 4(3):222–231 doi:10.1007/s10198–003–0182–5
Eick S, Pfister W, Korn-Stemme S, Magdefessel-Schmutzer U, Straube E (2000) Pathogen and resistance spectrum in intraoral infections of the jaw-facial area with special reference to anaerobic bacteria. Mund Kiefer Gesichtschir 4(4):234–239 doi:10.1007/PL00010788
Limeres J, Tomas I, Alvarez M, Diz P (2005) Empirical antimicrobial therapy for odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endo 100(3):263–264 doi:10.1016/j.tripleo.2004.12.019
Tomas I, Alvarez M, Limeres J, Otero JL, Saavedra E, Lopez-Melendez C et al (2004) In vitro activity of moxifloxacin compared to other antimicrobials against streptococci isolated from iatrogenic oral bacteremia in Spain. Oral Microbiol Immunol 19(5):331–335 doi:10.1111/j.1399–302x.2004.00167.x
Warnke PH, Becker ST, Springer IN, Harle F, Ullmann U, Russo PA et al (2008) ‘Grandmother penicillin’–not in vogue, but clinically still effective. J Antimicrob Chemother 61(4):960–962 doi:10.1093/jac/dkn030
Beckmann J, Kees F, Schaumburger J, Kalteis T, Lehn N, Grifka J et al (2007) Tissue concentrations of vancomycin and Moxifloxacin in periprosthetic infection in rats. Acta Orthop 78(6):766–773 doi:10.1080/17453670710014536
Malincarne L, Ghebregzabher M, Moretti MV, Egidi AM, Canovari B, Tavolieri G et al (2006) Penetration of moxifloxacin into bone in patients undergoing total knee arthroplasty. J Antimicrob Chemother 57(5):950–954 doi:10.1093/jac/dkl091
Metallidis S, Charokopos N, Nilolaidis J, Alexiadou E, Lazaraki G, Koumentaki E et al (2006) Penetration of moxifloxacin into sternal bone of patients undergoing routine cardiopulmonary bypass surgery. Int J Antimicrob Agents 28(5):428–432 doi:10.1016/j.ijantimicag.2006.08.019
Metallidis S, Topsis D, Nikolaidis J, Alexiadou E, Lazaraki G, Grovaris L et al (2007) Penetration of moxifloxacin and levofloxacin into cancellous and cortical bone in patients undergoing total hip arthroplasty. J Chemother 19(6):682–687
Financial interests
The authors have no commercial or political interests in the methodological or medical aspects presented in this paper. The investigation was partially granted by Bayer, the producer of Moxifloxacin. The grant was administered by the Department of Oral and Maxillofacial Surgery, University of Mainz.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Nawas, B., Walter, C., Morbach, T. et al. Clinical and microbiological efficacy of moxifloxacin versus amoxicillin/clavulanic acid in severe odontogenic abscesses: a pilot study. Eur J Clin Microbiol Infect Dis 28, 75–82 (2009). https://doi.org/10.1007/s10096-008-0587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0587-2