Abstract
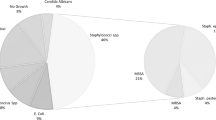
Responsible pathogens of chronic bone infections (CBI) are frequently resistant, requiring parenteral antimicrobial therapy. Therefore, adverse effects may be observed. We have determined the rate of adverse effects of antimicrobial therapy for CBI in a retrospective study of all patients receiving parenteral drugs via an implantable port. Patients from one medical ward (n = 89) and from one surgical ward (n = 40) between January 1995 and December 2005 were included in this study. The CBI included were 85 osteomyelitis (66%) and 44 prosthetic joint infections (34%). The main group of pathogens was Gram positive cocci (n = 144; 65%). The total duration of antibiotic treatment was 205 ± 200 days, including 133 ± 100 days for parenteral therapy. Thirty-three catheter-related complications were observed in 27 patients (21%). All complications led to hospitalization but none led to death. Twenty-one antibiotic-related complications occurred in 18 patients (16%), and one allergic reaction led to death. The mean duration of follow-up was 290 days. Remission was observed in 84 patients (65%). In multivariate analysis, adverse effects were mostly observed in the medical department. Adverse effects affect at least one third of the patients treated for CBI with parenteral antimicrobial therapy and are related to both the implantable port and the antibiotic compounds.
Similar content being viewed by others
References
Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi:10.1056/NEJMra040181
Bernard L, Hoffmeyer P, Assal M et al (2004) Trends in the treatment of orthopaedic prosthetic infections. J Antimicrob Chemother 53:127–129. doi:10.1093/jac/dkh033
Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364:369–379. doi:10.1016/S0140-6736(04)16727-5
Bernard L, El H, Pron B et al (2001) Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: evaluation of efficacy, tolerance and cost. J Clin Pharm Ther 26:445–451. doi:10.1046/j.1365-2710.2001.00380.x
Tice AD, Rehm SJ, Dalovisio JR et al (2004) Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis 38:1651–1672. doi:10.1086/420939
Hoffman-Terry ML, Fraimow HS, Fox TR et al (1999) Adverse effects of outpatient parenteral antibiotic therapy. Am J Med 106:44–49. doi:10.1016/S0002-9343(98)00362-3
Esposito S, Noviello S, Leone S et al (2004) Outpatient parenteral antibiotic therapy (OPAT) in different countries: a comparison. Int J Antimicrob Agents 24:473–478. doi:10.1016/j.ijantimicag.2004.06.004
Tice A (2001) The use of outpatient parenteral antimicrobial therapy in the management of osteomyelitis: data from the Outpatient Parenteral Antimicrobial Therapy Outcomes Registries. Chemotherapy 47(suppl 1):5–16. doi:10.1159/000048563
Galperine T, Ader F, Piriou P et al (2006) Outpatient parenteral antimicrobial therapy (OPAT) in bone and joint infections. Med Mal Infect 36:132–137. doi:10.1016/j.medmal.2006.01.002
Kurul S, Saip P, Aydin T (2002) Totally implantable venous-access ports: local problems and extravasation injury. Lancet Oncol 3:684–692. doi:10.1016/S1470-2045(02)00905-1
O’Grady NP, Alexander M, Dellinger EP et al (2002) Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol 23:759–769. doi:10.1086/502007
Nathwani D, Tice A (2002) Ambulatory antimicrobial use: the value of an outcomes registry. J Antimicrob Chemother 49:149–154. doi:10.1093/jac/49.1.149
Barbut F, Soukouna S, Lalande V et al (2004) Totally implantable venous access ports: frequency of complications and analysis of bacterial contamination after ablation. Pathol Biol (Paris) 52:566–574. doi:10.1016/j.patbio.2004.07.020
National Cancer Institute (2003) Cancer therapy evaluation program common terminology criteria for adverse events, version 3.0. Available at: http://ctep.cancer.gov/forms/CTCAEv3.pdf. Accessed on 10 October 2007
Talfer S, Conessa C, Herve S, Roguet E, de Rotalier P, Poncet JL (2003) Complications with totally implantable venous access device. A retrospective of 116 cases. Presse Med 32:1263–1268
Hartkamp A, van Boxtel AJ, Zonnenberg BA, Witteveen PO (2000) Totally implantable venous access devices: evaluation of complications and a prospective comparative study of two different port systems. Neth J Med 57:215–223. doi:10.1016/S0300-2977(00)00083-8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pulcini, C., Couadau, T., Bernard, E. et al. Adverse effects of parenteral antimicrobial therapy for chronic bone infections. Eur J Clin Microbiol Infect Dis 27, 1227–1232 (2008). https://doi.org/10.1007/s10096-008-0570-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0570-y