Abstract
Acinetobacter spp. are increasingly reported as important causes of human infection. Many isolates exhibit multi-drug resistance, raising concerns over our ability to treat serious infections with these organisms. The impact of infection on clinical outcome as well as the importance of multi-drug resistance is poorly defined. A descriptive retrospective observational study was undertaken of all episodes of Acinetobacter bacteremia occurring in a UK tertiary care centre from 1998–2006. Demographics of infected patients, characteristics and antimicrobial susceptibility of infecting strains were recorded and the impact of antimicrobial therapy on all causes of 30-day mortality assessed. Three hundred ninety-nine episodes of Acinetobacter bacteremia were identified, with A. baumannii being the most frequently isolated species. Most episodes occurred in critical care and were associated with multidrug resistance, with carbapenem resistance rising from 0% in 1998 to 55% in 2006. Although bacteremia due to carbapenem-resistant Acinetobacter and a requirement for critical care were associated with a higher mortality, mortality was not reduced by the administration of appropriate empirical antimicrobial therapy. A prospective study is required to identify both the most effective intervention and those most likely to benefit from treatment.
Similar content being viewed by others
Introduction
The genus Acinetobacter consists of a number of species of non-fermentative gram-negative rods within the family Moraxellaceae. These organisms are considered opportunistic pathogens, with A. baumannii in particular increasingly reported as an important cause of ventilator-associated pneumonia, bacteremia and sepsis in patients with burns, immunosuppression and critical illness. Much of the concern surrounding A. baumannii infection arises from the high rates of resistance to antimicrobial agents. Multidrug-resistant strains of A. baumannii (MRAB) resistant to all β-lactams, fluoroquinolones and aminoglycosides have been circulating in the UK since 2001. Although these are generally susceptible to the polymyxin fomulation colistin and the glycylcycline tigecycline [1], ‘pan-resistant’ isolates have been reported elsewhere [2]. Whilst much attention has focused on the mechanisms of antimicrobial resistance and the implications for infection control, the contribution of A. baumannii infection to morbidity and mortality remains poorly defined.
Isolation of Acinetobacter from wounds or respiratory samples may represent colonization rather than infection; Acinetobacter bacteremia may be a more useful indicator of true infection. Previous studies of Acinetobacter bacteremia have reported conflicting results. One retrospective study of A. baumannii bacteremia quoted a mortality of 22% [3], whilst a matched cohort study could find no significant attributable effect [4]. Some studies have also attempted to address the contribution of multidrug resistance. A study by Falagas et al. [5] suggested that inappropriate empirical antimicrobial therapy was independently associated with a poor clinical outcome. Although this study included a small number of MRAB strains susceptible only to polymyxin, there has been no large series involving infection with a common clone. Isolates susceptible only to polymyxin and tigecycline, typical of the prevalent UK MRAB strains, have been recovered from patients in our hospital since 2001. In view of the controversy regarding the clinical importance of Acinetobacter infection and the implications for empirical antibiotic regimens in the setting of endemic MRAB, we set out to undertake a retrospective analysis of all episodes of Acinetobacter bacteremia seen in our hospital over an 8-year period. The changing epidemiology, strain-dependent risk factors for infection as well as the role and impact of antimicrobial therapy for the treatment of MRAB are reviewed.
Materials and methods
Episodes of bacteremia were identified by a search of the clinical microbiology laboratory database (Barts and The London NHS Trust) from April 1998 to September 2006. This laboratory serves both the local community and a 973-bed teaching hospital. Anonymised demographic and laboratory data were collected from the electronic patient record and patient administration systems as available. Isolates were identified by biochemical profiling using API 20NE augmented from January 2005 by sequencing of the 16S rRNA gene when API 20NE gave unsatisfactory results. Isolates identified as members of the A. baumannii/calcoaceticus complex were grouped with A. baumannii. Those that could not reliably be identified were considered Acinetobacter spp. Antimicrobial susceptibility testing was performed by the British Society for Antimicrobial Chemotherapy (BSAC) disc diffusion method.
A retrospective observational study was conducted to determine the epidemiology, risk factors and outcome of Acinetobacter bacteremia. Data collected included infecting species, age, sex, co-morbid conditions, requirement for critical care and antimicrobial therapy administered. For a subgroup of patients nursed in intensive care with bacteremia due to A. baumannii, APACHE II scores were obtained from a review of the Intensive Care National Audit and Research Centre (ICNARC) database. The main outcomes were distribution of infecting species, 30-day mortality and impact of antimicrobial therapy.
Nosocomial infection was defined as infection occurring >48 h after admission. Isolation of Acinetobacter spp. from repeat blood cultures sets taken less than 48 h apart was considered as a single episode of bacteremia. Acinetobacter spp. isolated from repeat blood culture sets that differed in antimicrobial resistance profiles by ≥2 agents were considered as separate episodes of bacteremia. Isolation of Acinetobacter spp from repeat blood cultures sets taken >48 h apart and <5 days apart when the patient was receiving appropriate antimicrobial therapy was defined as ‘breakthrough bacteremia’. Isolation of Acinetobacter spp from a second episode of bacteremia in a patient receiving inappropriate therapy was defined as ‘persistent bacteremia’. A second episode of bacteremia occurring after the patient had received more than 5 days of appropriate therapy was defined as ‘relapse’. Multidrug resistance (MRAB) was defined as resistance to third-generation cephalosporins combined with aminoglycosides or quinolones; isolates also resistant to carbapenems were termed MRAB-C. Empirical antimicrobial therapy was defined as therapy started before the organism was identified in blood cultures or the sensitivities were not known. Therapy was also classified as either appropriate or inappropriate based on the in vitro susceptibility of the organism to the antimicrobial agents used.
Analysis of death within 30 days was restricted to adults, as no children (<18 years) died. Bacteremias more than 30 days after a previous bacteremia were considered to be independent of the presenting bacteremia; therefore, all analyses were restricted to the first episode of bacteremia. Stata 9 was used for all analyses (StataCorp. 2005 Stata Statistical Software: Release 9. College Station, TX: StataCorp LP).
Results and discussion
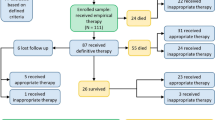
Three hundred ninety-nine episodes of Acinetobacter bacteremia were identified in 363 individuals, of which 368 were classified as independent. A large increase in the number of bacteremias was noted after the year 2000, with the numbers more than doubling between 1999–2000 and peaking in 2002 (Fig. 1). This represents an increase from 2 episodes per 100,000 bed days in 1998 to 18/100,000 in 2005. One hundred four (26%) of the episodes were considered community acquired, whilst 295 (74%) were nosocomial infections. One hundred eighty-seven isolates were identified as A. baumannii; 212 could not reliably be identified beyond the genus Acinetobacter. The greatest burden of A. baumannii bacteremia occurred in critical care (52%) followed by surgical (13%), general medical (10%) and oncology wards (7%). One hundred thirty-six isolates (34%) met the definition of multidrug resistance (MRAB), and 71 (52%) of these were also resistant to carbapenems (MRAB-C). Strains identified as A. baumannii were significantly more resistant than all other species to all antibiotics except colistin (p < 0.05). Resistance to carbapenems rose from 0% in 2000 to 55% in 2006 (Fig. 1) and was significantly associated with a requirement for critical care [p < 0.01; OR =4.81, 95% CI (2.73–8.48)]. A summary of the demographics and clinical characteristics of patients with A. baumannii versus Acinetobacter spp bacteremia is given in Table 1.
Male sex was a significant risk factor for MRAB-C bacteremia [p < 0.001; OR 3.39, 95% CI (1.76–6.54)]. Although the associations were not significant, the risk was higher for adult men in critical care (OR =4.46 vs. 1.67). All except one episode in critical care was associated with an indwelling catheter; outside of critical care the presence of an indwelling catheter also independently increased the risk, but not significantly [p = 0.08; OR 2.64, 95% CI (0.89–7.8)]. For the 120 patients nursed in intensive care, APACHE II scores were available for 43 individuals. The mean APACHE II score was 17 (range 7–36, SD ±7) and the mean length of stay prior to bacteremia in these individuals was 14 days (range 1–50, SD ±10).
Thirty-one bacteremias were repeat episodes. These were classified as persistent bacteremia due to inappropriate therapy (n = 7), breakthrough bacteremia (n = 2) and relapses (n = 22). Three patients relapsed twice, and one relapased three times.
Data on antibiotics administered were available for 209 patients. The most common regimens used were cephalosporins followed by quinolones and carbapenems. Appropriate empirical therapy was administered to only 43% of patients with A. baumannii bacteremia versus 61% of patients with Acinetobacter spp bacteremia. In patients with MRAB-C bacteremia, appropriate empirical therapy was given in only 23% of patients. Although appropriate empirical therapy was less likely to be administered for episodes in critical care [p = 0.08; OR 0.56, 95% CI (0.29–1.08)] when adjusted for carbapenem resistance, this was not significant [p = 0.53; OR 0.80, 95% CI (0.39–1.6)].
Twenty-five individuals died within 30 days of a bacteremia, 22 of which were within 30 days of the presenting episode. All deaths were in adults; risk and odds ratios of death were calculated for adults dying within 30 days of the initial episode. The crude risk of death was 7.4% [95% CI (4.7–10.9%)]. The risk of death in those with bacteremia due to Acinetobacter spp. was 3.9% [95% CI (1.1–9.6)] and 11.3% [95% CI (6.7–17.4)] in those with A. baumannii. In adults with MRAB-C the mortality rate was 16.4% [n = 9/55, 95% CI (7.8–28.8)] versus 5.4% [n = 13/243, 95% CI (2.9–9.0)] in those with carbapenem susceptible Acinetobacter bacteremia (Fig. 2a). The odds ratio for death within 30 days following bacteremia due to MRAB-C was significant [p = 0.007; OR =3.46, 95% CI (1.40–8.57)]. However, mortality following Acinetobacter bacteremia was significantly higher [14.6% versus 3.6%, p = 0.001; OR =4.58, 95% CI (1.80–11.6)] in patients nursed in intensive care (Fig. 2d) and, following adjustment for mortality associated with intensive care, the increased risk of death following MRAB-C bacteremia was not significant [p = 0.093; OR =2.27, 95% CI (0.87–5.93)]. Mortality may or may not have been influenced by empirical therapy. Although 30-day mortality was 8.0% (7/88) in those receiving appropriate therapy versus 13.5% (10/74) in those started on inappropriate antibiotics (Fig. 2b), this was not significant (p = 0.25). There was insufficient power in the study to detect an effect of empirical treatment in those with MRAB-C. However, 33% (2/6) of those given appropriate empirical treatment died compared with 27% (7/26) of those given inappropriate empirical treatment. All patients with MRAB-C who died after receiving appropriate empirical therapy were treated with a regimen that included colistin as the appropriate agent.
Survival of patients with Acinetobacter bacteremia; (a) carbapenem-resistant versus carbapenem susceptible strains (p < 0.01); (b) appropriate versus inappropriate antimicrobial therapy (not significant); (c) appropriate versus inappropriate therapy and intensive care treatment; (d) intensive care treatment versus no intensive care treatment (p < 0.01)
During the mid 1990s, carbapenem resistance emerged worldwide in the genus Acinetobacter. Coupled with this was a concomitant emergence of Acinetobacter spp. as a nosocomial pathogen, raising concerns over treatment options for this organism. As with the rest of the world, the prevalence of carbapenem resistant Acinetobacter has been increasing in the UK. In our institution resistance to carbapenems in blood culture isolates rose from 0% in 2001 to >50% in 2006. This overall increase coincides with the emergence of the MRAB-C OXA-23 clone-1 as the most prevalent strain in London and South East England. Analysis of isolates from our institution by the National Reference Laboratory since 2001 confirms the dominance of this clone [1].
The emergence of successful clones appears to have resulted in a shift in the distribution of Acinetobacter species associated with blood stream infection. In 1998 only one Acinetobacter isolate recovered from blood culture was speciated as A. baumannii; by 2002 this figure had risen to 47 and by 2006 A. baumannii accounted for 66% (n = 22) of all Acinetobacter bacteremias (Fig. 1). Although 124 isolates could still only be identified to the genus, improved identification of A. baumannii following the introduction of 16S sequence analysis post 2005 may account for some of the observed increase in this species. It should be emphasised that even using molecular methods Acinetobacter are notoriously difficult to speciate, accurate identification only being possible with reference methods such as ARDRA, AFLP and rpoB sequencing [6]. These taxonomic difficulties are an important factor to consider when comparing results across different studies.
Few studies have compared bacteremia due to A. baumannii with non-baumannii species, although a recent retrospective case-control study reported no difference in either clinical outcome or underlying patient demographics [7]. In contrast, we observed Acinetobacter spp bacteremia most frequently in children and in bacteremia presenting from the community. As non-baumannii species such as Acinetobacter lwoffii can be carried on healthy human skin [8], it is possible that these episodes represent pseudobacteremia or contamination of blood culture sets particulary in children where blood cultures may be difficult to take.
The majority of Acinetobacter bacteremias were nosocomial, caused by A. baumannii and associated with marked antimicrobial resistance. The unadjusted risk factors associated with A. baumannii bacteremia were the presence of an intravascular device and a requirement for critical care. In other studies of nosocomial Acinetobacter infection, advanced age, mechanical ventilation, renal failure and prolonged hospital stay have all been identified as important risk factors [9, 10]. It is possible that all these parameters are simply surrogate markers of underlying disease severity or a requirement for critical care. Controlling for the effects of individual treatments, e.g., activated protein C, tight glycaemic control and goal-directed therapy, is also difficult. However, the widespread introduction of ‘care bundles’ for the management of sepsis may be an opportunity to recruit patients in future studies who have at least all received the same standard of care. Another factor that may be important is control of the source of infection. Intravascular devices were identified as a potential source for Acinetobacter bacteremia in our study. Although the standard of care in our unit is to remove intravascular devices following an episode of gram-negative bacteremia, we were unable to assess compliance with this intervention retrospectively. There is a wealth of data on the benefits of device removal following bacteremia with other gram-negative bacteria, but few data on its impact on Acinetobacter bacteraemia. The importance of prompt removal of a colonised vascular access device as well as the impact of antimicrobial treatment should therefore be addressed.
Some authors have included the APACHE II score in the analysis of Acinetobacter clinical outcomes. As the APACHE II score is a parameter that in itself predicts mortality, it is not surprising that an elevated score in patients with A. baumannii bacteremia is associated with enhanced mortality [11]. If the APACHE II score is to be used in analyses of infections in intensive care, then cohorts should be matched as described by Blot et al. [12]. The only appropriately powered study we are aware of found that A. baumannii bacteremia did not contribute to death [4].
An ongoing concern is whether carbapenem resistance, as exhibited by MRAB-C strains, may have an effect on treatment outcomes. The development of carbapenem resistance clearly limits treatment options and may confer a selective advantage in an environment where carbapenems are extensively used. This has led some authors to propose that agents such as polymyxin, which retain activity against MRAB-C, be used empirically for the treatment of sepsis in clinical areas where MRAB-C are established [13]. We observed a significantly higher mortality amongst individuals infected with a carbapenem-resistant strain, a finding also reported in a recent study on mortality from MRAB-C bacteremia in Korea [14]. However, we did not observe any difference in mortality in patients who received appropriate or inappropriate empirical antimicrobial therapy. In patients with MRAB-C this was despite a delay in initiating appropriate therapy as 77% were treated empirically with an agent that was not active in-vitro. This suggests that ‘appropriate’ antimicrobial therapy is either ineffective or makes only a small contribution to the outcome of Acinetobacter bacteraemia.
In patients with MRAB-C the ‘appropriate’ agent used in all cases was the colistimethate sodium preparation of colistin, colomycin (Forest Laboratories, Kent, UK) given at the manufacturer's recommended dose of 1 million units every 8 h, equivalent to 240 mg of colistimethate sodium/day. There are very few data on the efficacy of colistin for the treatment of Acinetobacter infection, and concerns have been expressed over the pharmacokinetics of this agent in the treatment of severe gram-negative infections [15]. Our observation that ‘appropriate’ therapy did not influence mortality could reflect either inadequacy of colistin as a treatment for Acinetobacter bacteremia outright or that the dose selected was too low. Furthermore, although all isolates appeared susceptible to colistin by the BSAC disc diffusion method, the diffusion characteristics of colistin in agar are known to be unpredictable. The possibility that low levels of colistin resistance may not have been detected, or that colistin resistance developed during therapy, could also explain our findings [16]. Two patients who died following MRAB-C underwent treatment with colistin in combination with imipenem. Combinations of colistin with carbapenems, macrolides and rifampicin have been shown to be synergistic in-vitro versus some strains of MRAB, but not consistently against MRAB-C prevalent in the UK [17].
In summary this study describes changes in the epidemiology of Acinetobacter spp bacteremia in a large tertiary care centre. In particular it includes data on bacteremia due to MRAB-C, suggesting an association between multidrug resistance and poor outcome. This observation is complicated by the fact that most individuals with MRAB-C were nursed in intensive care, which was itself associated with heightened mortality. For bacteremia due to MRAB-C the finding that administration of appropriate empirical antimicrobial therapy did not influence mortality yet was associated with an increased risk of death is intriguing. It highlights the need for a prospective well-controlled trial to identify both those who require treatment and the most appropriate therapeutic regimen.
References
Coelho JM, Turton JF, Kaufmann ME, Glover J, Woodford N, Warner M, Palepou MF, Pike R, Pitt TL, Patel BC, Livermore DM (2006) Occurrence of carbapenem-resistant Acinetobacter baumannii clones at multiple hospitals in London and Southeast England. J Clin Microbiol 44(10):3623–3627
Manikal VM, Landman D, Saurina G, Oydna E, Lal H, Quale J (2000) Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin Infect Dis 31(1):101–106
Chen HP, Chen TL, Lai CH, Fung CP, Wong WW, Yu KW, Liu CY (2005) Predictors of mortality in Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect 38(2):127–136
Blot S, Vandewoude K, Colardyn F (2003) Nosocomial bacteremia involving Acinetobacter baumannii in critically ill patients: a matched cohort study. Intensive Care Med 29(3):471–475
Falagas ME, Kasiakou SK, Rafailidis PI, Zouglakis G, Morfou P (2006) Comparison of mortality of patients with Acinetobacter baumannii bacteraemia receiving appropriate and inappropriate empirical therapy. J Antimicrob Chemother 57(6):1251–1254
La Scola B, Gundi VA, Khamis A, Raoult D (2006) Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol 44(3):827–832
Choi SH, Choo EJ, Kwak YG, Kim MY, Jun JB, Kim MN, Kim NJ, Jeong JY, Kim YS, Woo JH (2006) Clinical characteristics and outcomes of bacteremia caused by Acinetobacter species other than A. baumannii: comparison with A. baumannii bacteremia. J Infect Chemother 12(6):380–386
Berlau J, Aucken H, Malnick H, Pitt T (1999) Distribution of Acinetobacter species on skin of healthy humans. Eur J Clin Microbiol Infect Dis 18(3):179–183
Grupper M, Sprecher H, Mashiach T, Finkelstein R (2007) Attributable mortality of nosocomial acinetobacter bacteremia. Infect Control Hosp Epidemiol 28(3):293–298
Sunenshine R, Wright M, Maragakis L, Harris A, Song X, Hebden J, Cosgrove S, Anderson A, Carnell J, Jernigan D, Kleinbaum D, Perl T, Standiford H, Srinivasan A (2007) Multidrug-resistant. Acinetobacter infection mortality rate and length of hospitalization. Emerging Infectious Diseases 13(1):97–103
Choi JY, Park YS, Kim CO, Park YS, Yoon HJ, Shin SY, Kim YA, Song YG, Yong D, Lee K, Kim JM (2005) Mortality risk factors of Acinetobacter baumannii bacteraemia. Intern Med J 35(10):599–603
Blot SI, Depuydt P, Annemans L, Benoit D, Hoste E, De Waele JJ, Decruyenaere J, Vogelaers D, Colardyn F, Vandewoude KH (2005) Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis 41(11):1591–1598
Falagas ME, Rafailidis PI (2007) When to include polymyxins in the empirical antibiotic regimen in critically ill patients with fever? A decision analysis approach. Shock 27(6):605–609
Kwon KT, Oh WS, Song JH, Chang HH, Jung SI, Kim SW, Ryu SY, Heo ST, Jung DS, Rhee JY, Shin SY, Ko KS, Peck KR, Lee NY (2007) Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother 59(3):525–530
Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL (2006) Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6(9):589–601
Tan TY, Ng LSY (2006) Comparison of three standardized disc susceptibility testing methods for colistin. J Antimicrob Chemother 58(4):864–867
Wareham DW, Bean DC (2006) In-vitro activity of polymyxin B in combination with imipenem, rifampicin and azithromycin versus multidrug resistant strains of Acinetobacter baumannii producing OXA-23 carbapenemases. Ann Clin Microbiol Antimicrob 5:10
Acknowledgements
This study was presented in part at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, 2006. No source of funding was available for this study. All authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wareham, D.W., Bean, D.C., Khanna, P. et al. Bloodstream infection due to Acinetobacter spp: epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis 27, 607–612 (2008). https://doi.org/10.1007/s10096-008-0473-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0473-y