Abstract
Background
Fibromyalgia syndrome is a widespread chronic pain condition identified by body-wide pain, fatigue, cognitive fogginess, and sleep issues. In the past decade, repetitive transcranial magnetic stimulation has emerged as a potential management tool.. In the present study, we enquired whether repetitive transcranial magnetic stimulation could modify pain, corticomotor excitability, cognition, and sleep.
Methods
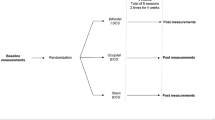
Study is a randomized, sham-controlled, double-blind, clinical trial; wherein after randomizing thirty-four fibromyalgia patients into active or sham therapy (n = 17 each), each participant received repetitive transcranial magnetic stimulation therapy. In active therapy was given at 1 Hz for 20 sessions were delivered on dorsolateral prefrontal cortex (1200 pulses, 150 pulses per train for 8 trains); while in sham therapy coil was placed at right angle to the scalp with same frequency. Functional magnetic resonance imaging was used to identify the therapeutic site. Pain intensity, corticomotor excitability, cognition, and sleep were examined before and after therapy.
Results
Baseline demographic and clinical parameters for both active and sham groups were comparable. In comparison to sham, active repetitive transcranial magnetic stimulation showed significant difference in pain intensity (P < 0.001, effect size = 0.29, large effect) after intervention. Other parameters of pain perception, cognition, and sleep quality also showed a significant improvement after the therapy in active therapy group only, as compared to sham.
Conclusions
Findings suggest that repetitive transcranial magnetic stimulation intervention is effective in managing pain alongside cognition and sleep disturbances in patients of fibromyalgia. It may prove to be an important tool in relieving fibromyalgia-associated morbidity.



Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and additional information can be provided if requested.
Abbreviations
- FMS:
-
Fibromyalgia Syndromes
- rTMS:
-
Repetitive Transcranial Magnetic Stimulation
- fMRI:
-
Functional Magnetic Resonance Imaging
- BOLD:
-
Blood Oxygen Level Dependent
- NeNa:
-
Neuronavigation
- MNI:
-
Montreal Imaging Institute
- ACR:
-
American College of Rheumatology
- MMSE:
-
Mini-Mental State Examination
References
Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F (2015) The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS ONE 10:0138024. https://doi.org/10.1371/journal.pone.0138024
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 62:600–610. https://doi.org/10.1002/acr.20140
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL (2016) 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 46:319–329. https://doi.org/10.1016/j.semarthrit.2016.08.012
Fitzcharles MA, Brachaniec M, Cooper L, Dubin R, Flynn T, Gerhold K (2017) A paradigm change to inform fibromyalgia research priorities by engaging patients and health care professionals. Can J Pain 1:137–147. https://doi.org/10.1080/24740527.2017.1374820
Häuser W, Ablin J, Perrot S, Fitzcharles MA (2017) Management of fibromyalgia: practical guides from recent evidence-based guidelines. Pol Arch Intern Med. 127:47–56. https://doi.org/10.20452/pamw.3877
Tiwari VK, Nanda S, Arya S, Kumar U, Sharma R, Kumaran SS (2021) Correlating cognition and cortical excitability with pain in fibromyalgia: a case control study. Adv Rheumatol 61:10. https://doi.org/10.1186/s42358-021-00163-x
Arnold LM, Clauw DJ, McCarberg BH (2011) Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc 86:457–464. https://doi.org/10.4065/mcp.2010.0738
Bell T, Trost Z, Buelow MT, Clay O (2018) Younger J, Moore D: Meta-analysis of cognitive performance in fibromyalgia. J Clin Exp Neuropsychol 40:698–714. https://doi.org/10.1080/13803395.2017.1422699
Wu Y-LL, Huang C-JJ, Fang S-CC, Ko L-HH, Tsai P-SS (2018) Cognitive impairment in fibromyalgia: a metaanalysis of case-control studies. Psychosom Med 80:432–438. https://doi.org/10.1097/PSY.0000000000000575
Lawson K (2020) Sleep dysfunction in fibromyalgia and therapeutic approach options. OBM Neurobiol 4:16. https://doi.org/10.21926/obm.neurobiol.2001049
Carville SF, Arendt-Nielsen L, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC (2008) EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum 67:536–541. https://doi.org/10.1136/ard.2007.071522
Calandre EP, Rico-Villademoros F, Slim M (2017) Pharmacological treatment of fibromyalgia: Is the glass half empty or half full? Pain Manag 7:5–10. https://doi.org/10.2217/pmt-2016-0044
Calandre EP, Rico-Villademoros F, Rodríguez-López CM (2012) Monotherapy or combination therapy for fibromyalgia treatment? Curr Rheumatol Rep 14:568–575. https://doi.org/10.1007/s11926-012-0278-y
O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM (2018) Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev 16:008208. https://doi.org/10.1002/14651858.CD008208.pub4
Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V (2020) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol 131:474–528. https://doi.org/10.1016/j.clinph.2019.11.002
Knijnik LM, Dussán-Sarria JA, Rozisky JR, Torres IL, Brunoni AR, Fregni F (2016) Repetitive transcranial magnetic stimulation for fibromyalgia: systematic review and meta-analysis. Pain Pract 16:294–304. https://doi.org/10.1111/papr.12276
Saltychev M, Laimi K (2017) Effectiveness of repetitive transcranial magnetic stimulation in patients with fibromyalgia: a meta-analysis. Int J Rehabil Res 40:11–18. https://doi.org/10.1097/MRR.0000000000000207
Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR (2013) Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS. Neuropsychopharmacol 38:1189–1197. https://doi.org/10.1038/npp.2013.1312of14
Seminowicz DA, Moayedi M (2017) The dorsolateral prefrontal cortex in acute and chronic pain. J Pain 18:1027–1035. https://doi.org/10.1016/j.jpain.2017.03.008
Sampson SM, Rome JD, Rummans TA (2006) Slow-frequency rTMS reduces fibromyalgia pain. Pain Med 7:115–118. https://doi.org/10.1111/j.1526-4637.2006.00106.x
Lee SJ, Kim DY, Chun MH, Kim YG (2012) The effect of repetitive transcranial magnetic stimulation on fibromyalgia: a randomized sham-controlled trial with 1-mo follow-up. Am J Phys Med Rehabil 91:1077–1085. https://doi.org/10.1097/PHM.0b013e3182745a04
Tanwar S, Mattoo B, Kumar U, Bhatia R (2020) Repetitive transcranial magnetic stimulation of the prefrontal cortex for fibromyalgia syndrome: A randomised controlled trial with 6-months follow up. Adv Rheumatol 60:34. https://doi.org/10.1186/s42358-020-00135-7
Wassermann EM (1998) Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation. Electroencephalogr Clin Neurophysiol 108:1–6. https://doi.org/10.1016/S0168-5597(97)00096-8
Tiwari V, Nanda S, Arya S, Kumar U, Bhatia R (2020) Effect of repetitive transcranial magnetic stimulation in male patients of fibromyalgia. Indian J Rheumatol 15:134. https://doi.org/10.4103/injr.injr_163_19
Goetz SM, Murphy DL, Peterchev AV (2014) Transcranial magnetic stimulation device with reduced acoustic noise. IEEE Magn Lett 5:14. https://doi.org/10.1109/LMAG.2014.2351776
Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V et al (2012) A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol 123:858–882. https://doi.org/10.1016/j.clinph.2012.01.010
Chipchase L, Schabrun S, Cohen L, Hodges P, Ridding M, Rothwell J (2012) A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: An international consensus study. Clin Neurophysiol 123:1698–1704. https://doi.org/10.1016/j.clinph.2012.05.003
Neggers SFW, Langerak TR, Schutter DJLG, Mandl RCW, Ramsey NF (2004) A stereotactic method for imageguided transcranial magnetic stimulation validated with fMRI and motor-evoked potentials. Neuroimage 21:1805–1817. https://doi.org/10.1016/j.neuroimage.2003.12.006
Jensen MP, McFarland CA (2020) Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 55:195–203. https://doi.org/10.1016/0304-3959(93)90148-I
Sharma S, Palanchoke J, Reed D, Abbott JH (2017) Translation, cross-cultural adaptation and psychometric properties of the Nepali versions of numerical pain rating scale and global rating of change. Health Qual Life Outcomes 15:236. https://doi.org/10.1186/s12955-017-0812-8
Melzack R (1975) The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1:277–299. https://doi.org/10.1016/0304-3959(75)90044-5
Shroff RA, Dabholkar TY (2021) The hindi version of mcgill pain questionnaire: A cross-cultural adaptation study in rheumatoid arthritis. Indian J Rheumatol 16(2):159-S163. https://doi.org/10.4103/injr.injr_194_20
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental status”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Ganguli M, Ratcliff G, Chandra V, Sharma S, Gilby J, Pandav R (1995) A hindi version of the MMSE: The development of a cognitive screening instrument for a largely illiterate rural elderly population in india. Int J Geriatr Psychiatry 10:367–377. https://doi.org/10.1002/gps.930100505
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662. https://doi.org/10.1037/h0054651
Algom D, Chajut E (2019) Reclaiming the stroop effect back from control to input-driven attention and perception. Front Psychol 10:1683. https://doi.org/10.3389/fpsyg.2019.01683
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213. https://doi.org/10.1016/0165-1781(89)90047-4
Bajpai G, Shukla G, Pandey R, Gupta A, Afsar M, Goyal V (2016) Validation of a modified Hindi version of the Epworth sleepiness scale among a north Indian population. Ann Indian Acad Neurol 19:499–504. https://doi.org/10.4103/0972-2327.194427
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545. https://doi.org/10.1093/sleep/14.6.540
Manzar MD, Moiz JA, Zannat W, Spence DW, Pandi-Perumal SR, Bahammam AS (2015) Validity of the Pittsburgh sleep quality index in Indian university students. Oman Med J 30:193–202. https://doi.org/10.5001/omj.2015.41
Bilir I, Askin A, Sengul I, Tosun A (2021) Effects of high-frequency neuronavigated repetitive transcranial magnetic stimulation in fibromyalgia syndrome: a double-blinded, randomized controlled study. Am J Phys Med Rehabil 100:138–146. https://doi.org/10.1097/PHM.0000000000001536
Andrade C, Arumugham SS, Thirthalli J (2016) Adverse effects of electroconvulsive therapy. Psychiatr Clin North Am 39:513–530. https://doi.org/10.1016/j.psc.2016.04.004
Martin DM, McClintock SM, Forster JJ, Lo TY, Loo CK (2017) Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depress Anxiety 34:1029–1039. https://doi.org/10.1002/da.22658
Cao X, Deng C, Su X, Guo Y (2018) Response and remission rates following high-frequency versus low-frequency repetitive transcranial magnetic stimulation (rTMS) over right DLPFC for treating major depressive disorder (MDD): a meta-analysis of randomized, double-blind trials. Front Psychiatry 9:413. https://doi.org/10.3389/fpsyt.2018.00413
Baudic S, Attal N, Mhalla A, de Andrade DC, Perrot S, Bouhassira D (2013) Unilateral repetitive transcranial magnetic stimulation of the motor cortex does not affect cognition in patients with fibromyalgia. J Psychiatr Res 47:72–77. https://doi.org/10.1016/j.jpsychires.2012.09.003
Jiang CG, Zhang T, Yue FG, Yi ML, Gao D (2013) Efficacy of repetitive transcranial magnetic stimulation in the treatment of patients with chronic primary insomnia. Cell Biochem Biophys 67:169–173. https://doi.org/10.1007/s12013-013-9529-4
Park EJ, Lee SJ, Do Yle Koh YM (2014) Repetitive transcranial magnetic stimulation to treat depression and insomnia with chronic low back pain. Korean J Pain 27:285–289. https://doi.org/10.3344/kjp.2014.27.3.28513of14
Ahdab R, Ayache SS, Brugières P, Farhat WH, Lefaucheur JP (2016) The hand motor hotspot is not always located in the hand knob: a neuronavigated transcranial magnetic stimulation study. Brain Topogr 29:590–597. https://doi.org/10.1007/s10548-016-0486-2
Mylius V, Ayache SS, Ahdab R, Farhat WH, Zouari HG, Belke M (2013) Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: inter-rater reliability, accuracy, and influence of gender and age. Neuroimage 78:224–232. https://doi.org/10.1016/j.neuroimage.2013.03.061
Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, Haynor D (2009) More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry 66:509–515. https://doi.org/10.1016/j.biopsych.2009.04.03414[1]
Brighina F, Curatolo M, Cosentino G, De Tommaso M, Battaglia G, Sarzi-Puttini PC, Guggino G, Fierro B (2019) Brain Modulation by Electric Currents in Fibromyalgia: A Structured Review on Non-invasive Approach With Transcranial Electrical Stimulation. Front Hum Neurosci 13:40
Acknowledgements
We would like to acknowledge Department of Physiology, All India Institute of Medical Sciences, New Delhi – 110029; for providing me various resources for my thesis work. A sincere acknowledgement to Department of NMR, All India Institute of Medical Sciences, New Delhi – 110029; for neuroimaging techniques and analysis of its data .
Funding
No funding was provided that could have influenced the outcome of the study.
Author information
Authors and Affiliations
Contributions
The manuscript is a collective work of all the authors. The research concept was thesis work of MD student, VKT, involved in protocol writing, patient recruitment, execution of all tests, result analysis and preparation of the manuscript; SN was involved in performing tests, manuscript writing; SC did analysis of fMRI data; AK helped in data analysis and preparation of manuscript; RS contributed in execution of the cognitive evaluation of patients; UK contributed in diagnosis and referring the patients to our test lab, manuscript writing; SK for performing fMRI and related data analysis; RB was chief guide with original idea, over all planning, preparation of protocol, execution of the study and manuscript writing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study was approved on 06 September 2017 by Institute Ethics Committee, All India Institute of Medical Sciences, New Delhi, India (Ref. No. IEC-450/01.09.2017). All the participants had given written consent to participate in the study and they were allowed to quite at any time during the study.
Conflict of interest
No potential conflict of interest was relevant to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tiwari, V.K., Kumar, A., Nanda, S. et al. Effect of neuronavigated repetitive Transcranial Magnetic Stimulation on pain, cognition and cortical excitability in fibromyalgia syndrome. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07317-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07317-x