Abstract
Background
Parkinson’s disease (PD) presents with motor symptoms that hinder physical activity. This study aimed to thoroughly investigate swallowing dysfunction in patients with PD using videofluoroscopy (VF) and the Movement Disorder Society (MDS)-Unified PD Rating Scale (UPDRS) sub-scores.
Methods
This study was part of an intervention project to evaluate the effectiveness of cervical percutaneous interferential current stimulation in patients with Hoehn and Yahr stages 2–4 PD. Baseline data, including swallowing-related indicators such as VF, were obtained and compared to the MDS-UPDRS sub-scores including rigidity, tremor, postural instability/gait difficulty, and limb scores.
Results
Twenty-seven patients were included in this study. In the VF analysis, laryngeal penetration/aspiration, oral cavity residue, epiglottic vallecular residue, and pharyngeal residue were observed with remarkable frequency. The multivariate analysis revealed that the mean rigidity score of UPDRS was an independent and significantly correlated factor with laryngeal penetration/aspiration during the ingestion of 10 mL of water (odds ratio 1.294, 95% confidence interval 1.035–1.617; p = 0.024).
Conclusion
This study revealed a correlation between muscle rigidity and laryngeal penetration or aspiration risk. The detailed comparative analysis of various individual PD symptoms and swallowing disorders was substantial, which enabled early detection of the risk of swallowing disorder and the implementation of appropriate measures.
Trial registration number
jRCTs062220013.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurological disorders frequently result in dysphagia, increasing susceptibility to aspiration pneumonia and significantly affecting patient prognosis and outcomes. This leads to increased medical and caregiving costs. Parkinson’s disease (PD) is one of the most prevalent neurological disorders, and its incidence is rapidly increasing globally [1]. Aspiration pneumonia is the principal cause of mortality in patients with PD. Consequently, evaluating and formulating suitable and effective interventions for preventing pneumonia is paramount. PD causes several swallowing disorders, including anomalies in the oral cavity, pharyngeal transport [2], delayed swallowing reflex [3], and residual pharyngeal material [4].
Primary symptoms of PD include bradykinesia, muscular rigidity, and tremors [5]. Various clinical manifestations have been reported, and PD is now understood to be a systemic disorder encompassing non-motor symptoms as well. A heterogeneous array of diverse manifestations is observed even when considering only motor symptoms. Consequently, clinical symptoms, disease progression, and associated issues differ significantly among patients with PD. The complexity of swallowing difficulties is believed to arise from this diversity; thus it is difficult to evaluate this phenomenon comprehensively. Nevertheless, research investigating the relationship between the motor symptoms of PD and swallowing difficulties is exceedingly limited.
We are currently conducting a project investigating the effect of cervical percutaneous electrical interferential current stimulation on swallowing in PD [6]. Interferential current stimulation activates peripheral nerves in the throat without discomfort [7]. This approach potentially alleviates dysphagia and has shown positive effects on saliva production [8], swallowing frequency, and airway sensitivity [7]. As a conceptual foundation of this exploratory research, we investigated the correlation between the subtypes of motor symptoms in PD and swallowing dysfunction using videofluoroscopy (VF), the gold standard evaluation method.
This study aimed to thoroughly investigate swallowing dysfunction in PD by comparing VF swallowing results to the Movement Disorder Society (MDS)-Unified PD Rating Scale (UPDRS) subscores, which assess the severity of various PD symptoms [9].
Materials and methods
Ethics approval, registrations, and patient consent
This research was approved by the Certified Review Board of Hiroshima University (Hiroshima, Japan) (approval number: CRB6180006) and conducted in accordance with the national government’s regulations following the Helsinki Declaration of 1964. The study was registered with jRCT (jRCTs062220013). Written informed consent was obtained from all participating patients.
Study design and protocol
This study comprised cross-sectional exploratory research that is part of the project in which we investigated the effect of cervical percutaneous electrical interferential current stimulation on swallowing. The project protocol has been published previously [6]. This study was conducted at the Hiroshima University Hospital. We enrolled patients who were clinically diagnosed with probable or confirmed PD according to the Movement Disorder Society criteria [5] and possessed the ability to provide informed consent. Additionally, they were required to be at Hoehn and Yahr stages 2–4 at the time of enrollment and provide written informed consent. The inclusion criteria were as follows: receiving consistent levodopa dose for > 1 month and age > 19 years < 86 years. Individuals meeting any of the following criteria were not considered for the study: having a pacemaker or implantable defibrillator; undergoing deep brain stimulation; currently pregnant or attempting to conceive; and a diagnosis or history of head or neck cancer, active pneumonia, or past swallowing rehabilitation. Participants’ motor symptoms and swallowing dynamics were examined using baseline data collected before an intervention.
Videofluoroscopy
An X-ray imaging device (Ultimax-i; Canon Medical System Corporation, Tochigi, Japan) was used to conduct the tests while the patients were seated. The trials involved 3 and 10 mL of water mixed with 30%w/w barium contrast medium (Barytester A240 Powder ®, Fushimi Pharmaceutical Co. Ltd., Kagawa, Japan). The patients were instructed to ingest this mixture, delivered via a syringe directly to the floor of the mouth. Employing an X-ray system, we captured images from various angles: forward, towards the lips, backward to the pharyngeal wall, upward to the nasal cavity, and downwards to the upper esophageal sphincter. The recordings were obtained at a rate of 30 frames/s and stored on a DVD. To prevent fatigue phenomena, these investigations outlined here were conducted at the beginning of the videofluoroscopy, with breaks taken to prevent consecutive swallows.
Three experienced dentists (A Hiraoka, A Haruta, and MY), blinded to patient information, evaluated the videofluorographic recordings. Their expertise was in assessing the presence or absence of laryngeal penetration/aspiration and identifying the clearance or prevalence of residue in the oral cavity, vallecular area, and pharynx following a single swallow.
Additionally, they measured the time the bolus took to pass through specific anatomical landmarks and performed a temporal analysis. Two key parameters were calculated: laryngeal elevation delay time (LEDT) and pharyngeal delay time (PDT). LEDT refers to the duration between the bolus tip reaching the vallecula and the peak laryngeal elevation. An established LEDT threshold was set at 0.32 s [10]. In contrast, PDT, is the duration from when the bolus tip reaches the intersection of the lower border of the mandible and the base of the tongue until laryngeal elevation begins. In healthy adults, this interval ranges from 0 to 0.2 s [11].
Furthermore, we assessed whether there was a delay in the swallowing reflex. This was defined as the liquid remaining in the pyriform sinuses for more than 0.1 s (equivalent to 3 frames) before being swallowed [12]. The three observers engaged in thorough discussions and reached a consensus for each observation and measurement.
Data acquisition
Two neurologists (MN and HY) performed the clinical evaluation and diagnosis. The recorded data included body mass index (BMI), grip strength, disease duration, alcohol consumption, smoking habits, MDS-UPDRS score [9], medication, Functional Oral Intake Scale (FOIS) score, Eating Assessment Tool-10 (EAT-10) score, and blood test values [13]. Previously reported methods were used to investigate cough reflex, tongue pressure, and peak expiratory flow [14,15,16]. The levodopa equivalent daily dose (LEDD) was calculated according to a recent study [17]. All evaluations were conducted in the On state. By referring to a previous study [18], data were extracted and calculated separately for the following components from the MDS-UPDRS Part 3 to analyze motor symptom subtypes: the mean value of the rigidity (3.3 a–e), tremor (3.15–3.18), postural instability and gait difficulty (PIGD) (3.9–3.13), and limb (3.4–3.8) scores.
Statistical analyses
Data are expressed as mean ± standard deviation or median (minimum, maximum) for continuous variables and frequencies and percentages for discrete variables. Univariate analyses were conducted using methods such as single regression analysis, contingency tables, analysis of variance, and logistic regression analysis to investigate the data related to the findings of VF for swallowing 3 and 10 mL of water, as well as temporal analyses. In this process, we included the mean values of rigidity scores, tremor scores, PIGD scores, and limb scores, and as mentioned, UPDRS Part 3, in the analyses. Subsequently, we explored the associations between various VF findings and the corresponding UPDRS subscores. Initially, we conducted univariate analyses and extracted factors with p-values < 0.10. These factors were then subjected to a multivariate analysis. Statistical analyses were performed using JMP statistical software version 16 (SAS Institute Inc., Cary, NC, USA). Appropriate statistical tests, such as the χ2 test, Mann–Whitney U test, or unpaired t-test, were employed to assess intergroup variances. Statistical significance was set at p < 0.05.
Results
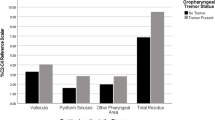
In this study, 27 participants were enrolled, and baseline data were obtained. The participant demographics are presented in Table 1. Additionally, detailed data, including temporal analysis of the VF conducted by swallowing 3 and 10 mL of water, are listed in Table 2. Laryngeal penetration/aspiration, oral cavity, epiglottic vallecular, and pharyngeal residues were found at a remarkable frequency. Although there was a slightly higher frequency of abnormal findings during swallowing 10 mL of water than during swallowing 3 mL of water, no statistically significant difference was observed. However, LEDT and PDT were almost within the normal range when swallowing 3 mL of water, in which the number of patients who exhibited deviations from the standard values of LEDT and PDT were five and two, respectively. Although an unpaired t-test indicated no statistically significant difference for swallowing 10 mL compared to 3 mL of water, a trend of extended LEDT was observed. The number of patients who had deviations from the standard values of laryngeal LEDT and PDT were 14 and 2, respectively. Using the χ2 test, the number of patients with prolonged LEDT when swallowing 10 mL of water was significantly higher than that when swallowing 3 mL of water (p = 0.010). However, LEDT was not associated with the UPDRS subscores, including the neck rigidity subscore. For swallowing 3 mL of water, five patients (18.5%) had swallowing reflex delays, and for swallowing 10 mL of water, four (14.8%) had swallowing reflex delays. However, this was not frequent.
An investigation into the correlation between VF findings during the ingestion of 3 and 10 mL of water and the patient's background revealed that for swallowing 10 mL of water, a connection was observed between the mean value of rigidity scores and laryngeal penetration/aspiration. Following the univariate analysis, the factors associated with laryngeal penetration/aspiration (p < 0.10) included BMI, UPDRS Part 3, and the mean value of the rigidity scores. To address the confounding effects of the UPDRS Part 3 and the mean value of rigidity scores, separate multivariate analyses involving BMI were conducted. Consequently, the mean value of rigidity scores was identified as an independent and significantly correlated factor with laryngeal penetration/aspiration during the ingestion of 10 mL of water (odds ratio 1.294, 95% confidence interval 1.035–1.617, p = 0.024) (Table 3). No significant association was observed between LEDT and UPDRS scores or their subscores, including the neck rigidity subscore.
Discussion
Patients with PD exhibit a diverse range of neurological symptoms, including motor manifestations. Swallowing difficulties among patients with PD also vary. This study examined the correlation between swallowing and each subscore for the main motor symptoms in patients with PD. The results indicated that the frequency of laryngeal penetration or aspiration was increased with increasing muscular rigidity severity.
This study demonstrated that muscle rigidity was associated with a higher risk of aspiration than other motor symptoms; however, this tendency was not observed during swallowing 3 mL of water but rather during swallowing 10 mL of water. No significant results were obtained regarding the effect of swallowing smaller amounts of water. Furthermore, during the swallowing of 10 mL of water, an increasing number of patients with PD exhibited delayed laryngeal elevation to the peak. LEDT refers to the duration between the bolus tip reaching the vallecula and peak laryngeal elevation [10]. This phenomenon was also pronounced when the amount of water swallowed was greater. Although a significant correlation with UPDRS subscores was not observed, it is plausible that prolonging laryngeal elevation time during the swallowing of larger volumes, owing to the bradykinesia and muscle rigidity characteristic of PD was a consistent outcome. Therefore, patients with PD and pronounced muscle rigidity should be particularly cautious during swallowing and avoid a larger bolus amount.
PD symptoms are categorized into limb and axial symptoms, with axial symptoms strongly associated with falls and swallowing difficulties [19]. In this study, we also examined the PIGD subscore extracted from UPDRS Part 3; however, no significant correlation was found. Previous studies have reported a relationship between falls and swallowing difficulties [19]; although, discrepancies in the results could be attributed to differences in scoring methods. Nevertheless, in this study, the advantage was extracting scores from the UPDRS, the gold standard for PD assessment, and conducting swallowing evaluations using VF.
Conversely, no significant correlations were found between UPDRS scores, including subscores, and measures such as tongue pressure and peak expiratory flow. Some systematic reviews and meta-analyses suggest relations among age, tongue pressure, and hand grip [20, 21]. In conditions such as stroke, amyotrophic lateral sclerosis, and sarcopenia, strong correlations have been observed between disease severity scores and tongue pressure, which are also significantly associated with aspiration and pneumonia development [14, 22, 23]. However, these conditions are also associated with paralysis. PD generally does not lead to paralysis but presents with smooth movement impairment primarily characterized by bradykinesia, making strength evaluation methods insufficient for assessing aspiration risk. Exploring and developing simple screening methods and combining these approaches are considered crucial tasks in addressing swallowing evaluation and PD management.
This study had several limitations. First, the study was conducted at a single site, warranting subsequent investigations across multiple facilities and a larger sample size. Second, a notable challenge arose from the limited patient pool available for scrutiny in this intervention-oriented project. The primary focus was on cervical interferential current stimulation to enhance the cough reflex assessment, which dictated the sample size based on prior research and existing literature. Nonetheless, to conduct a comprehensive scrutiny and analysis of swallowing disorders in patients with diverse PD symptoms, a considerable patient cohort must be included. Therefore, further patient recruitment is required to facilitate future research.
Conclusion
This study demonstrated a correlation between muscle rigidity and aspiration risk. The detailed comparative analysis of various individual symptoms of PD and swallowing disorders was substantial. The findings of this study can contribute to a thorough understanding of swallowing disorders in patients with PD and the possibility of precise interventions; thus, further research is warranted.
Data availability
The data supporting the findings of this study are available from the corresponding author upon request.
References
Dorsey ER, Sherer T, Okun MS, Bloem BR (2018) The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 8:S3–S8. https://doi.org/10.3233/JPD-181474
Logemann J, Blonsky ER, Boshes B (1973) Lingual control in Parkinson’s disease. Trans Am Neurol Assoc 98:276–278
Robbins JA, Logemann JA, Kirshner HS (1986) Swallowing and speech production in Parkinson’s disease. Ann Neurol 19:283–287. https://doi.org/10.1002/ana.410190310
Bushmann M, Dobmeyer SM, Leeker L, Perlmutter JS (1989) Swallowing abnormalities and their response to treatment in Parkinson’s disease. Neurology 39:1309–1314. https://doi.org/10.1212/wnl.39.10.1309
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Nakamori M, Toko M, Yamada H, Hayashi Y, Yoshikawa K, Yoshikawa M et al (2023) Impact of neck percutaneous interferential current sensory stimulation on swallowing function in patients with Parkinson’s disease: a single-arm, open-label study protocol. Contemp Clin Trials Commun 33:101158. https://doi.org/10.1016/j.conctc.2023.101158
Furuta T, Takemura M, Tsujita J, Oku Y (2012) Interferential electric stimulation applied to the neck increases swallowing frequency. Dysphagia 27:94–100. https://doi.org/10.1007/s00455-011-9344-2
Hasegawa Y, Sugahara K, Sano S, Sakuramoto A, Kishimoto H, Oku Y (2016) Enhanced salivary secretion by interferential current stimulation in patients with dry mouth: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol 121:481–489. https://doi.org/10.1016/j.oooo.2016.01.017
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P et al (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170. https://doi.org/10.1002/mds.22340
Miyaji H, Umezaki T, Adachi K, Sawatsubashi M, Kiyohara H, Inoguchi T et al (2012) Videofluoroscopic assessment of pharyngeal stage delay reflects pathophysiology after brain infarction. Laryngoscope 122:2793–2799. https://doi.org/10.1002/lary.23588
Kim Y, McCullough GH, Asp CW (2005) Temporal measurements of pharyngeal swallowing in normal populations. Dysphagia 20:290–296. https://doi.org/10.1007/s00455-005-0029-6
Nakamori M, Hosomi N, Imamura E, Matsushima H, Maetani Y, Yoshida M et al (2021) Association between stroke lesions and videofluoroscopic findings in acute stroke patients. J Neurol 268:1025–1035. https://doi.org/10.1007/s00415-020-10244-4
Crary MA, Mann GD, Groher ME (2005) Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 86:1516–1520. https://doi.org/10.1016/j.apmr.2004.11.049
Hiraoka A, Yoshikawa M, Nakamori M, Hosomi N, Nagasaki T, Mori T et al (2017) Maximum tongue pressure is associated with swallowing dysfunction in ALS patients. Dysphagia 32:542–547. https://doi.org/10.1007/s00455-017-9797-z
Nakamori M, Imamura E, Kuwabara M, Ayukawa T, Tachiyama K, Kamimura T et al (2020) Simplified cough test can predict the risk for pneumonia in patients with acute stroke. PLoS ONE 15:e0239590. https://doi.org/10.1371/journal.pone.0239590
Kamimura T, Nakamori M, Naito H, Aoki S, Nezu T, Imamura E et al (2023) Peak expiratory flow, but not tongue pressure, can predict pneumonia development in older adults. Eur Geriatr Med 14:211–217. https://doi.org/10.1007/s41999-023-00744-7
Jost ST, Kaldenbach MA, Antonini A, Martinez-Martin P, Timmermann L, Odin P et al (2023) Levodopa dose equivalency in Parkinson’s disease: updated systematic review and proposals. Mov Disord 38:1236–1252. https://doi.org/10.1002/mds.29410
Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC (2013) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord 28:668–670. https://doi.org/10.1002/mds.25383
Umemoto G, Furuya H (2020) Management of dysphagia in patients with Parkinson’s disease and related disorders. Intern Med 59:7–14. https://doi.org/10.2169/internalmedicine.2373-18
Arakawa I, Igarashi K, Imamura Y, Müller F, Abou-Ayash S, Schimmel M (2021) Variability in tongue pressure among elderly and young healthy cohorts: a systematic review and meta-analysis. J Oral Rehabil 48:430–448. https://doi.org/10.1111/joor.13076
Arakawa-Kaneko I, Watarai Y, Schimmel M, Abou-Ayash S (2022) Relationship between tongue pressure and handgrip strength: a systematic review and meta-analysis. J Oral Rehabil 49:1087–1105. https://doi.org/10.1111/joor.13362
Nakamori M, Hosomi N, Ishikawa K, Imamura E, Shishido T, Ohshita T et al (2016) Prediction of pneumonia in acute stroke patients using tongue pressure measurements. PLoS ONE 11:e0165837. https://doi.org/10.1371/journal.pone.0165837
Nakamori M, Hosomi N, Takaki S, Oda M, Hiraoka A, Yoshikawa M et al (2016) Tongue thickness evaluation using ultrasonography can predict swallowing function in amyotrophic lateral sclerosis patients. Clin Neurophysiol 127:1669–1674. https://doi.org/10.1016/j.clinph.2015.07.032
Funding
Masahiro Nakamori received grants from the Grants-in-Aid for Scientific Research (21K17512), Tsuchiya Memorial Medical Foundation, Japanese Society of Dysphagia Rehabilitation, Mitsui Sumitomo Insurance Welfare Foundation, and Casio Science Promotion Foundation. Yukio Mikami received grants from Grants-in-Aid for Scientific Research (20K11188) and the Ministry of Health, Labor, and Welfare of Japan (20GA1001, 23GA2001). Hirofumi Maruyama received grants from Grants-in-Aid for Scientific Research (23H02827).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This research was approved by the Certified Review Board of Hiroshima University (Hiroshima, Japan) (approval number: CRB6180006) and was conducted in accordance with the national government’s regulations following the Helsinki Declaration of 1964. The study was registered with jRCT (jRCTs062220013). Written informed consent was obtained from all participating patients.
Competing interests
Hirofumi Maruyama received honoraria from Eisai, Shionogi, Otsuka Pharmaceutical, and Sumitomo Pharma.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakamori, M., Toko, M., Yamada, H. et al. Association between motor symptoms of Parkinson’s disease and swallowing disorders. Neurol Sci 45, 2021–2026 (2024). https://doi.org/10.1007/s10072-023-07238-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07238-1