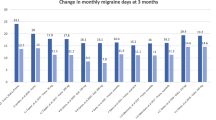
Abstract
Chronic migraine (CM) is a great challenge for physicians dealing with headaches. Despite the introduction of the monoclonal antibodies (mAbs) acting against the calcitonin gene-related peptide (CGRP) that has revolutionized the treatment of CM, some patients still experience an incomplete relief. So, the association of two preventive treatments may be a reliable option for these patients. So, onabotulinumtoxinA (BT-A) and anti-CGRP mAbs may be used together, and some pre-clinical and clinical evidence of an additive action of the 2 drugs is emerging. In particular, since BT-A acts mainly on C-fibers and anti-CGRP mAbs on Aδ ones, their association may prevent the wearing-off phenomenon of BT-A, thus giving an additional benefit in those patients experiencing an incomplete response to BT-A alone. Despite this, the clinical studies available in the literature have a small sample size, often a retrospective design, and are heterogeneous in terms of the outcomes chosen. Considering this, the evidence of a favorable effect of the association between BT-A and anti-CGRP mAbs is still scarce. Furthermore, this association is explicitly forbidden by many National regulatory agencies, due to the high costs of both treatments. Anyway, their association could help in reducing the burden associated with the most severe cases of CM, thus relieving the direct and indirect costs of this condition. More well-designed studies with big samples are needed to unveil the real therapeutic gain of this association. Moreover, pharmacoeconomics studies should be performed, to assess the economic suitability of this association.
Similar content being viewed by others
Data availability
The authors take full responsibility for the data, the analysis, and interpretation of the research, and they have full access to all of the data. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- BBB:
-
Blood-brain barrier
- BT-A:
-
OnabotulinumtoxinA
- CGRP:
-
Calcitonin gene-related peptide
- CGRPr:
-
Calcitonin gene-related peptide receptor
- CM:
-
Chronic migraine
- mAb:
-
Monoclonal antibody
- MMA:
-
Middle meningeal artery
- MOH:
-
Medication-overuse headache
- PREEMPT:
-
Phase III REsearch Evaluating Migraine Prophylaxis Therapy
- SNAP-25:
-
Synaptosomal associated protein of 25 kDa (SNAP-25)
- TG:
-
Trigeminal ganglion
- TNC:
-
Trigeminal nucleus caudalis
- TRPV1:
-
Transient receptor potential vanilloid type 1
- TRPA1:
-
Transient receptor potential cation channel subfamily A member 1
References
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. https://doi.org/10.1177/0333102417738202
Burch RC, Buse DC, Lipton RB (2019) Migraine: epidemiology, burden, and comorbidity. Neurol Clin 37:631–649. https://doi.org/10.1016/j.ncl.2019.06.001
Safiri S, Pourfathi H, Eagan A, Mansournia MA, Khodayari MT, Sullman MJM, Kaufman J, Collins G, Dai H, Bragazzi NL, Kolahi AA (2022) Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain 163:e293-2309. https://doi.org/10.1097/j.pain.0000000000002275
Agostoni EC, Barbanti P, Calabresi P, Colombo B, Cortelli P, Frediani F, Geppetti P, Grazzi L, Leone M, Martelletti P, Pini LA, Prudenzano MP, Sarchielli P, Tedeschi G, Russo A, the Italian chronic migraine group (2019) Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain 20:92. https://doi.org/10.1186/s10194-019-1038-4
Sacco S, Russo A, Geppetti P, Grazzi L, Negro A, Tassorelli C, Tedeschi G, Martelletti P (2020) What is changing in chronic migraine treatment? An algorithm for onabotulinumtoxinA treatment by the Italian chronic migraine group. Expert Rev Neurother 20:1275–1286. https://doi.org/10.1080/14737175.2020.1825077
Rothrock JF, Adams AM, Lipton RB, Silberstein SD, Jo E, Zhao X, Blumenfeld AM, FORWARD Study investigative group (2019) FORWARD study: evaluating the comparative effectiveness of OnabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. Headache 59:1700–1713. https://doi.org/10.1111/head.13653
Drellia K, Kokoti L, Deligianni C, Papadopoulos D, Mitsikostas DD (2021) Anti-CGRP monoclonal antibodies for migraine prevention: a systematic review and likelihood to help or harm analysis. Cephalalgia 41:851–864. https://doi.org/10.1177/0333102421989601
Sacco S, Braschinky M, Ducros A, Lampl C, Little P, Maassen van den Brink A, Pozo-Rosich P, Reuter U, Ruiz de la Torre E, Sanchez del Rio M, Sinclair AJ, Katsarava Z, Martelletti P (2020) European headache federation consensus on the definition of resistant and refractory migraine: developed with the endorsement of the European Migraine and Headache Alliance (EMHA). J Headache Pain. 21:76. https://doi.org/10.1186/s10194-020-01130-5
Torres-Ferrus M, Gallardo VJ, Alpuente A, Caronna E, Gine-Cipres E, Pozo-Rosich P (2021) The impact of anti-CGRP monoclonal antibodies in resistant migraine patients: a real-world evidence observational study. J Neurol 268:3789–3798. https://doi.org/10.1007/s00415-021-10523-8
D’Amico D (2012) Controversies in migraine: monotherapy. Neurol Sci 33:141–145. https://doi.org/10.1007/s10072-012-1059-0
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH (2017) Optimal database combination for literature searches in systematic review: a prospective exploratory study. Syst Rev 6:245. https://doi.org/10.1186/s13643-017-0644-y
Edvinsson JCA, Viganò A, Alekseeva A, Alieva E, Arruda R, De Luca C, D’Ettore N, Frattale I, Kurnukhina M, Macerola N, Malenkova E, Maiorova M, Nivokova A, Rehulka P, Rapaccini V, Rosh,china O, Vanderschueren G, Zvaune L, Andreou AP, Haanes KA (2020) European Headache Federation School of Advanced Studies (EHF-SAS). The fifth cranial nerve in headaches. 21:65. 13.https://doi.org/10.1186/s10194-020-01134-1
Noseda R, Melo-Carrillo A, Rony-Reuven N, Strassman AM, Burstein R (2019) Non-trigeminal nociceptive innervation of the posterior dura: implications to occipital headache. J Neurosci 39(1867–1880):14. https://doi.org/10.1523/JNEUROSCI.2153-18.2018
Levy D, Labastida-Ramirez A, MaassenVanDenBrink A (2019) Current understanding of meningeal and cerebral vascular function underlying migraine headache. Cephalalgia 39:1606–1622. https://doi.org/10.1177/033102418771350
Burstein R, Blake P, Schain A, Perry C (2017) Extracranial origin of headache. Curr Opin Neurol 30:263–271
Busch V, Jakob W, Juergens T, Schulte-Mattler W, Kaube H, May A (2006) Functional connectivity between trigeminal and occipital nerves revealed by occipital nerve blockade and nociceptive blink reflexes. Cephalalgia 26:50–55. https://doi.org/10.1111/j.1468-2982.2005.00992.x
Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K (2008) Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: difference between peripheral and central CGRP receptor distribution. J Comp Neurol 507:1277–1299. https://doi.org/10.1002/cne.21607
Iyengar S, Johnson KW, Ossipov MH, Aurora SK (2019) CGRP and the trigeminal system in migraine. Headache 59:659–681. https://doi.org/10.1111/head.13529
Burstein R, Blumenfeld AM, Silberstein SD, Manack AA, Brin MF (2020) Mechanism of action on onabotulinumtoxinA in chronic migraine: a narrative review. Headache 60:1259–1272
Burstein R, Yamamura H, Malick A, Strassman AM (1998) Chemical stimulation of the intracranial dural induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 79:964–982
Burstein R, Cutrer MF, Yarnitsky D (2000) The development of cutaneous allodynia during a migraine attack: clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 123:1703–1709
Blumenfeld A, Durham PL, Feoktistov A, Hay DL, Russo AF, Turner I (2021) Hypervigilance, allostatic load, and migraine prevention: antibodies to CGRP or receptor. Neurol Ther 10:469–497. https://doi.org/10.1007/s40120-021-00250-7
Wattiez AS, Wang M, Russo AF (2019) CGRP in animal models of migraine. Handb Exp Pharmacol 255:85–107. https://doi.org/10.1007/164_2018_187
Ashina H, Schytz HW, Ashina M (2018) CGRP in human models of primary headache. Cephalalgia 38:353–360. https://doi.org/10.1177/0333102416684344
Waskito A, Yamamoto Y, Raman S, Kano F, Yan H, Raju R, Afroz S, Morita T, Ikutame D, Okura K, Oshima M, Yamamoto A, Baba O, Matsuka Y (2021) Peripherally administered botulinum toxin type A localizes bilaterally in trigeminal ganglia of animal model. Toxin (Basel) 13:704. https://doi.org/10.3390/toxins13100704
Matak I, Riederer P, Lackovic Z (2012) Botulinum toxin’s axonal transport from periphery to the spinal cord. Neurochem Int 61:236–239. https://doi.org/10.1016/j.neuint.2012.05.001
Matak I, Back-Rojecky L, Filipovic B, Lackovic Z (2011) Behavioural and immunoistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience 186:201–207. https://doi.org/10.1016/j.neuroscience.2011.04.026
Durham PL, Cady R, Cady R (2004) Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache 44:35–42. https://doi.org/10.1111/j.1526-4610.2004.04007.x
Shimizu T, Shibata M, Toriumi H, Iwashita T, Funakubo M, Sato H, Kuroi T, Ebine T, Koizumi K, Suzuki N (2012) Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol Dis 48:367–378. https://doi.org/10.1016/j.nbd.2012.07.010
Zhang X, Strassman AM, Novack V, Brin MF, Burstein R (2016) Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors’ responses to stimulation of TRPV1 and TRPA1 channels: are we getting closer to solving this puzzle? Cephalalgia 36:875–886. https://doi.org/10.1177/0333102416636843
Burstein R, Zhang XC, Levy D, Aoki RK, Brin MF (2014) Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia 34:853–869. https://doi.org/10.1177/0333102414527648
Filipovic B, Matak I, Bach-Rojecky L, Lackovic Z (2012) Central action of peripherally applied botulinum toxin type A on pain and dural protein extravasation in rat model of trigeminal neuropathy. PLoS One 7:e29803. https://doi.org/10.1371/journal.pone.0029803
Lackovic Z, Filipovic B, Matak I, Helyes Z (2016) Activity of botulinum toxin type A in cranial dura: implications for treatment of migraine and other headaches. Br J Pharmacol 173:279–291. https://doi.org/10.1111/bph.13366
Melo-Carrilo A, Strassman AM, Schain AJ, Noseda R, Ashina S, Adams A, Brin MF, Burstein R (2019) Exploring the effects of extracranial injections of botulinum toxin type A on prolonged intracranial meningeal nociceptors responses to cortical spreading depression in female rats. Cephalalgia 39:1258–1365. https://doi.org/10.1177/0333102419873675
Noma N, Watanabe K, Sato Y, Imamura Y, Yamamamoto Y, Ito R, Maruno M, Shimizu K, Iwata K (2017) Botulinum neurotoxin type A alleviates mechanical hypersensitivity associated with infraorbital nerve constriction injury in rats. Neurosci Lett 637:96–101. https://doi.org/10.1016/j.neulet.2016.11.043
Matak I, Rossetto O, Lackovic Z (2014) Botulinum toxin type A selectivity for certain types of pain is associated with capsaicin-sensitive neurons. Pain 155:1516–1526. https://doi.org/10.1016/j.pain.2014.04.027
Kitamura Y, Matsuka Y, Spigelman I, Ishihara Y, Yamamoto Y, Sonoyama W, Kamioka H, Yamashiro T, Kuboki T, Oguma K (2009) Botulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience 159:1422–1429. https://doi.org/10.1016/j.neuroscience.2009.01.066
Noseda R, Schain AJ, Melo-Carrillo A, Tien J, Stratton J, Mai F, Strassman AM, Burstein R (2020) Fluorescently-labeled fremanezumab is distributed to sensory and autonomic ganglia and the dura but not to the brain of rats with uncompromised blood brain barrier. Cephalalgia 40:229–240. https://doi.org/10.1177/0333102419896760
Melo-Carrillo A, Noseda R, Nir RR, Schain AJ, Stratton J, Strassman AM, Burstein R (2017) Selective inhibition of trigeminovascular neurons by fremanezumab: a humanized monoclonal anti-CGRP antibody. J Neurosci 37:7149–7163. https://doi.org/10.1523/JNEUROSCI.0576-17.2017
Melo-Carrillo A, Strassman AM, Nir RR, Schain AJ, Noseda R, Stratton J, Burstein R (2017) Fremanezumab-a humanized monoclonal anti-CGRP antibody-inhibits thinly myelinated (Aδ) but not unmyelinated (C9 meningeal nociceptors. J Neurosci 37:10587–10596. https://doi.org/10.1523/JNEUROSCI.2211-17.2017
Ohlsson L, Kronvall E, Stratton J, Edvinsson L (2018) Fremanezumab blocks CGRP induced dilatation in human cerebral, middle meningeal and abdominal arteries. J Headache Pain 19:66. https://doi.org/10.1186/s10194-018-0905-8
Grell AS, Hanes KA, Johansson SE, Edvinsson L, Sams A (2019) Fremanezumab inhibits vasodilatatory effects of CGRP and capsaicin in rat cerebral artery-potential role in conditions of severe vasoconstriction-. Eur J Pharmacol 864:172726. https://doi.org/10.1016/j.ejphar.2019.172726
Zeller J, Poulsen KT, Sutton JE, Abdiche YN, Collier S, Chopra R, Garcia CA, Pons J, Rosenthal A, Shelton DL (2008) CGRP function-blocking antibodies inhibit neurogenic vasodilation without affecting heart rate or arterial blood pressure in the rat. Br J Pharmacol 155:1093–1103. https://doi.org/10.1038/bjp.2008.334
Schain AJ, Melo-Carrillo A, Stratton J, Strassman AM, Burstein R (2019) CSD-induced arterial dilatation and plasma protein extravasation are unaffected by fremanezumab. implication for CGRP’s role in migraine with aura. J Neurosci 39:6001–6011. https://doi.org/10.1523/JNEUROSCI.0232-19.2019
Christensen SL, Petersen S, Kristensen DM, Olesen J (2019) Munro G- Targeting CGRP via receptor antagonism and antibody neutralization in two distinct rodent models of migraine-like pain. Cephalalgia 39:1827–1837. https://doi.org/10.1177/0333102419861726
Toni T, Tamanaha R, Newman B, Liang Y, Lee J, Carrazana E, Vajjala V, Viereck J, Liow KK (2021) Effectiveness of dual migraine therapy with CGRP inhibitors and onabotulinumtoxinA injections: case series. Neurol Sci 42:5373–5376. https://doi.org/10.1007/s10072-021-05547-x
Cohen F, Armand C, Lipton RB, Vollbracht S (2021) Efficacy and tolerability of calcitonin gene-related peptide-targeted monoclonal antibody medications as add-on therapy to onabotulinumtoxinA in patients with chronic migraine. Pain Med 22:1857–1863. https://doi.org/10.1093/pm/pnab093
Mechtler L, Saikali N, McVige J, Hughes O, Traut A, Adams AM (2022) Real-world evidence for the safety and efficacy of CGRP monoclonal antibody therapy added to onabotulinumtoxinA treatment for migraine prevention in adult patients with chronic migraine. Front Neurol 12:788159. https://doi.org/10.3389/fneur.2021.788159
Silvestro M, Tessitore A, Scotto di Clemente F, Battista G, Tedeschi G, Russo A (2021) Additive interaction between onabotulinumtoxin-A and erenumab in patients with refractory migraine. Front Neurol 12:656294. https://doi.org/10.3389/fneur.2021.656294
Blumenfeld AM, Frishberg BM, Schim JD, Iannone A, Schneider G, Yedigarova L, Adams AM (2021) Real-world evidence for control of chronic migraine patients receiving CGRP monoclonal antidoby therapy added to onabotulinumtoxinA: a retrospective chart review. Pain Ther 10:809–826. https://doi.org/10.1007/s40122-021-00264-x
Armanious M, Khalil N, Lu Y, Jimenez-Sanders R (2020) Erenumab and onabotulinumtoxinA combination therapy for the prevention of intractable chronic migraine without aura: a retrospective analysis. J Pain Palliat Care Pharmacother 35:1–6. https://doi.org/10.1080/15360288.2020.1829249
Boudreau GP (2020) Treatment of chronic migraine with erenumab alone or as an add on therapy: a realworld observational study. Anesth Pain Res 4(1–4):25
Ozudogru SN, Bartell JW, Yuan H, Digre KB, Baggaley SK (2020) The effect of adding calcitonin gene-related peptide monoclonal antibodies to onabotulinum toxin A therapy on headache burden: a retrospective observational case series. Headache 60:1442–1443
Lu J, Zhang Q, Guo X, Liu W, Xu C, Hu X, Ni J, Lu H, Zhao H (2021) Calcitonin gene-related peptide monoclonal antibody versus botulinum toxin for the preventive treatment of chronic migraine: evidence from indirect treatment comparison. Front Pharmacol 12:631204. https://doi.org/10.3389/fphar.2021.631204
Pellesi L, Do TP, Ashina H, Ashina M, Burstein R (2020) Dual therapy with anti-CGRP monoclonal antibodies and botulinum toxin for migraine prevention: is there a rationale? Headache 60:1056–1065. https://doi.org/10.1111/head.13843
Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L (2013) Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain 14:1289–1303. https://doi.org/10.1016/j.jpain.2013.03.010
Edvinsson JCA, Warfringe K, Krause DN, Blixt FW, Sheykhzade M, Edvinsson L, Haanes KA (2019) C-fibers may modulate adjacent Adelta-fibers through axon-axon CGRP signaling at nodes of Ranvier in the trigeminal system. J Headache Pain 20:65. https://doi.org/10.1186/s10194-019-1055-3
Eftekhari S, Edvinsson L (2011) Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci 12:112. https://doi.org/10.1186/1471-2202-12-112
Masters-Israilov A, Robbins MA (2019) OnabotulinumtoxinA wear-off phenomenon in the treatment of chronic migraine. Headache 59:1753–1761. https://doi.org/10.1111/head.13638
Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB (2015) Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 35:478–488. https://doi.org/10.1177/0333102414547138
Orlando V, Mucherino S, Monetti VM, Trama U, Menditto E (2020) Treatment patterns and medication adherence among newly diagnosed patients with migraine: a drug utilisation study. BMJ Open 10:e038972. https://doi.org/10.1136/bmjopen-2020-038972
Guerzoni S, Pellesi L, Baraldi C, Cainazzo MM, Negro A, Martelletti P, Pini LA (2017) Long-term treatment benefits and prolonged efficacy of onabotulinumtoxinA in patients affected by chronic migraine and medication overuse headache over 3 years of therapy. Front Neurol 8:586. https://doi.org/10.3389/fneur.2017:00586
Iannone LF, Fattori D, Benemei S, Chiarugi A, Geppetti P, De Cesaris F (2022) Long-term effectiveness of three anti-CGRP monoclonal antibodies in resistant chronic migraine patients based on the MIDAS score. CNS Drugs 36:191–202
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the draft of the manuscript. All authors read and approved the last version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflictof Interest
S. G. received travel grants and honorary from Allergen, Novartis, Teva, and Ely Lilly. C. B. received travel grants and honorary from Allergen, Novartis, Teva, and Ely Lilly. L. P. is the Chief Scientific Officer of EDRA-LSWR Publishing Company and of Inpeco SA Total Lab Automation Company. In the last year, he has been a scientific consultant to AbbVie, USA; BCG, Switzerland; Boehringer-Ingelheim, Germany; Compass Pathways, UK; Johnson & Johnson, USA; Takeda, USA; VeraSci, USA; Vifor, Switzerland.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guerzoni, S., Baraldi, C. & Pani, L. The association between onabotulinumtoxinA and anti-CGRP monoclonal antibodies: a reliable option for the optimal treatment of chronic migraine. Neurol Sci 43, 5687–5695 (2022). https://doi.org/10.1007/s10072-022-06195-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06195-5