Abstract
Background
Multiple sclerosis (MS) accounts for 176 cases per 100,000 inhabitants (female/male ratio = 2:1) in Italy. For most of the patients (67%), the disease course is relapsing–remitting MS (RRMS).
Objective
To compare the costs and quality-adjusted life years (QALYs) of teriflunomide in RRMS naïve patients vs. RRMS patients previously treated (experienced) with other disease-modifying therapies in Italy.
Methods
A four health states Markov model-supported cost-utility analysis (CUA) covering a 7-year timespan through annual cycles was developed, following the healthcare sector and the societal viewpoints. Part of the parameters that populated the Markov model was obtained from a questionnaire administered to four primary Italian MS centres. Costs of healthcare and non-healthcare resources, expressed in euro (€) 2019, and QALYs were discounted at 3% real social discount rate. One-way, scenario and probabilistic sensitivity analyses tested the uncertainty of the baseline findings.
Results
Baseline CUA shows that teriflunomide in RRMS naïve patients is strongly dominant vs. experienced patients (healthcare sector perspective: − €1042.68 and + 0.480 QALYs; societal perspective: − €6782.81 and + 0.480 QALYs). Sensitivity analyses confirmed the robustness of the baseline results.
Conclusion
Teriflunomide in RRMS naïve vs. experienced patients is cost-effective and possibly strongly dominant from both the healthcare sector and the society viewpoints in Italy. Our findings need further confirmation from real-world studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic debilitating neurological disorder, accounting for 176 cases per 100,000 inhabitants (female/male ratio = 2:1) in Italy [1, 2].
For most of the patients (67%), the disease course is relapsing–remitting MS (RRMS) [2].
MS implies a sustained consumption of healthcare resources, reduces patients’ productivity, worsens her/his health-related quality of life (utility) [3] and often requires informal care [3].
In recent years, the treatment paradigms of MS have profoundly changed, due to the availability of a wide spectrum of disease modifying therapies (DMTs), acting on the immune system with distinct mechanisms of action. These also include oral therapies, such as teriflunomide, dimethyl-fumarate, fingolimod, cladribine often used in RRMS patients; thanks to their proven efficacy and safety, also being able to enhance patients’ compliance [4].
From a recent survey of the literature, the oral agents appear to be generally well tolerated with an acceptable safety profile [5]. However, this does not exclude that there may be more subtle and specific issues with each of the different drugs [5].
Most patients with MS switch between DMTs during their lifetime because non-responders or for safety concerns. The choice of the optimal treatment has therefore become increasingly complex, having as its first goal the ability to prevent the accumulation of disability over time [6].
As it was proved to reduce relapses and delay disability progression [7,8,9], since 2014 teriflunomide (Aubagio® — Sanofi Srl, Italy) 14 mg per os once per day has been reimbursed by the Italian National Health Service (INHS) as first line DMT for RRMS [10]. However, it was quite often used after the patient had already tried a different DMT, even for convenience reasons [11, 12].
The development of new drugs in MS has led to an increase in costs for the management of the disease. In this scenario, economic evaluation of healthcare programmes, in addition to clinical indication, plays a relevant role in supporting resource allocation decision-making.
This study presents a cost-utility analysis (CUA) [3] (Supplementary Information-SI Definition 1) that, adopting both the healthcare sector and the societal viewpoints, aims at verifying whether privileging the use of teriflunomide in RRMS patients who did not receive any previous therapy (naïve) vs. RRMS patients who had been already treated (experienced) with up to three DMTs is also cost-effective in Italy.
Materials and methods
Markov model
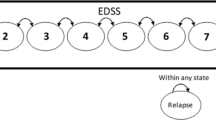
CUA was supported by a Markov model with four mutually exclusive and exhaustive health states (controlled RRMS: health state when patient enters the model; RRMS relapse; RRMS remission; all-cause mortality) (Fig. SI1) [3, 13, 14].
The Markov model lasts 7 years (i.e. 7 1-year Markov cycles) as it does not consider the possible conversion from RRMS to secondary progressive (SP) MS, since teriflunomide has no therapeutic indication for SPMS [10].
At each Markov cycle, two hypotethical cohorts of 1000 RRMS naïve and 1000 RRMS experienced patients remain in the same Markov state, progress to another Markov state or die for age- and gender-specific all-cause mortality, according to a transition probability matrix calculated on a subset of 721 parameters detailed separately (Tables SI1-SI56) [3, 13, 14].
Parts of the clinical, demographic and economic parameters that populated the Markov model (266/721 = 36.89%) were obtained from a questionnaire [15] administered by in-presence (50%) or phone (50%) interviews during June 2019 to a convenient sample [16] of 4 neurologists expert in MS (approximately 5000 patients followed-up per year in total) representative of as many primary MS centres in the north, middle and south Italy, as well as from research assumptions and literature.
The Expanded Disability Status Scale (EDSS) score-specific probability of relapse was obtained from the literature [17, 18].
The probability of remission after a clinical relapse was set at 20% (Table SI31) [19].
According to neurologists’ qualified opinion [15], from year 2 onwards, 10% RRMS naïve patients who relapsed were assumed to switch to second line therapies as alemtuzumab, cladibrine, fingolimod, natalizumab or ocrelizumab due to teriflunomide ineffectiveness (Table SI39).
Adherence to teriflunomide that was expected to influence both cost and quality-adjusted life years (QALYs) [3] was assumed 100% for both the hypothetical cohorts of patients.
For each Markov state, QALYs were calculated by multiplying life-years saved (LYS) by utility (Table SI38) [3]. Utility values related to EDSS score and disutility values for possible adverse events (AEs) related to teriflunomide were retrieved from previous researches [17, 20,21,22,23,24].
As this research did not imply patients’enrollment, no ethics committee approval of the study protocol (included the abovementioned questionnaire) was required by the Italian legislation [25].
The Markov model was developed with software Microsoft® Excel® for Windows® 2010 (Microsoft Corporation, Seattle, WA, USA).
Healthcare and non-healthcare resources identification, quantification and valuation
Both healthcare and non-healthcare resources were considered. Healthcare resources included teriflunomide and other DMTs (alemtuzumab; cladribine; fingolimod; natalizumab; ocrelizumab); for alemtuzumab, natalizumab, ocrelizumab, healthcare professionals’ time, drugs and disposables for premedication, administration and postemedication in outpatient or day-hospital setting; patients assessment before and during treatment with teriflunomide and other DMTs; drugs and healthcare procedures to manage relapses and possible AEs due to teriflunomide; patients follow-ups (with and without relapses); mobility aids (walking canes; walkers; crutches; wheelchairs) (Tables SI1-SI19; SI23-SI30; SI32-SI37; SI39-SI49).
Being the same for both the hypothetical cohorts of patients, diagnosis-related healthcare resources were not considered.
Teriflunomide and other DMTs were costed at ex-factory price (net of mandatory discounts) [10, 26] as they are administered in hospital setting only; other drugs were costed at retail price as they can be purchased at territorial pharmacy.
Healthcare procedures were valued according to the INHS tariffs for day-hospital and outpatient setting, which were assumed to be good proxies for their opportunity cost (i.e. the cost of the best forgone alternative) (Tables SI20; SI50-SI52) [27].
Usually, INHS tariffs for drugs administration include the cost of drugs. Since teriflunomide and other DMTs were valued separately, cost related to their administration in day-hospital or outpatient setting was halved to avoid double counting (i.e. costing the same resource twice) [3, 24].
The yearly cost of disability aids was obtained from the literature (Table SI52).
Non-healthcare resources included car transportation back and forth between home and hospital; car parking at the hospital; patient and caregiver’s time (working hours lost for patients aged < 70; leisure hours lost for patients aged ≥ 70 and for all the caregivers) for transportation, assessments, therapy or follow-ups; patients’ home and car adaptations (Tables SI9; SI13; SI22; SI48; SI49).
Non-healthcare resources were valued via unit monetary standards (Table SI52). The loss of working and leisure hours was costed via the gross wage rate (net wage + income taxes + social security contributions) and take-home wage rate (net wage only) approaches, respectively [28]. The loss of working hours experienced by housekeepers affected by RRMS was valued at homehelper hourly gross wage [3].
Resources were grouped into two different cost categories [3]. Healthcare sector costs included all the healthcare resources funded by INHS or hospital. Patient and/or her/his family costs included all non-healthcare resources plus healthcare resources not funded by INHS (e.g. over the counter drugs for hair thinning and rachialgy) (Tables SI21; SI52).
Costs were expressed in euros (€) at 2019 values and inflated to that year when necessary.
Costs, LYS and QALYs were discounted at 3% real social discount rate [3, 29] and half-cycle correction was applied (i.e. death was assumed to occurr half-way between the annual Markov cycle, so that dead notional patients were assigned 6-month costs, LYS and QALYs) [13, 14].
Statistical analysis
The number of notional patients in each Markov state was reported as mean and standard deviation (SD).
The majority of the parameters that populated the Markov model (438/721 = 60.75%) were assigned a theoretical probability distribution [14].
The beta distribution was fitted to dichotomous events (i.e. events that imply 2 pathways), such as probabilities and RRMS stage-specific utility values.
Polytomous events (i.e. events that imply ≥ 3 pathways), such as conditional probabilities of switching to other DMTs given the ineffectiveness of teriflunomide, followed a Dirichlet distribution.
The gamma distribution was fitted to volume of healthcare resources consumption (if different from drugs posology) and EDSS scores.
The normal distribution was assigned to the unit cost of healthcare resources different from drugs.
Point estimate and 95% confidence interval (95% CI) were calculated for all the parameters which were given a statistical distribution [14].
Parameter standard error was determined analytically or by imposing an appropriate coefficient of variation on the sample mean (Tables SI40-SI52) [14].
For parameters which were not assigned a theoretical probability distribution point estimate and range were reported.
No hypothesis test was performed on costs, LYS and QALYs totalled by RRMS naïve or experienced patients.
Sensitivity analyses
One-way (OW), scenario (S) and probabilistic (P) sensitivity analyses (SA) tested the robustness of the baseline ICUR [3].
Parameters included in OWSA were varied one at a time, holding the others at their base case values [3]; parameter baseline point estimates were replaced with the limits of the range or the 95% CI of the assigned theoretical probability distributions [14, 24].
OWSA explored the variations in ICUR due to changes in all the parameters related to event probabilities; resource consumption; unit cost; utility and disutility values (Tables SI40-SI52).
The 3% baseline real social discount rate was also changed (0%; 5%) [29] to check its influence on the base case findings.
OWSA results are displayed as departures from the basecase ICUR on a tornado chart and in tables.
The first SSA provided the annual point estimates of the ICUR during the 7-year timespan the Markov model stretches over [3], in order to investigate the relationship between ICUR and time and its potential bearing on the cost-effectiveness profile of the healthcare technologies under comparison.
The second and third SSA assessed the impact on cost and QALYs due to a lower adherence probability to teriflunomide (from 90 to 50%) and a higher probability of recovery after RRMS relapse (from 30 to 90%) for both the hypothetical cohorts of patients.
PSA explored the parameters joint uncertainty via a 10,000-iteration Monte Carlo simulation [3, 14]. For each Monte Carlo iteration, a random value was drawn for each one of the 438 parameters which were fitted a statistical distribution (the remaining 283 parameters entered the PSA at their base case estimate), so that 10,000 ICURs were simulated [3, 14].
An algebraic manipulation of the ICUR (net monetary benefit (NMB)) (SI Definition 2) supported the construction of cost-effectiveness acceptability curve (CEAC) and cost-effectiveness acceptability frontier (CEAF) (SI Definitions 3 and 4) [3, 14, 30, 31].
Averaging over the results of the Monte Carlo simulation, PSA showed the probability that one of the alternatives was cost-effective (CEAC) or optimal (CEAF), that is having the highest expected NMB vs. comparator for different threshold values [3, 14, 30, 31].
CEAC and CEAF overlap if and only if the healthcare programme showing the higher probability of being cost-effective has also the highest expected NMB [3, 14, 30, 31].
As recommended by literature, posology, number of administrations and unit costs of drugs were excluded from SA [3, 14].
Results
Markov model
On average, RRMS naïve enter the model at 33 years (range: 25–49), being younger than RRMS-experienced notional patients (37 years; range: 25–48). The first administration of teriflunomide occurs after 6 years (range: 1–13) and 11 years (range: 8–13) from diagnosis for RRMS naïve and experienced notional patients, respectively.
Female patients were 78.71% and 77.08% for RRMS naïve and experienced notional patients, respectively.
During the 7-year timespan (Table 1),
the RRMS naïve hypothetical cohort reports, on average, 291 (SD: 115) non-relapsed patients (29.09% of the starting 1000 notional patients), 511 (SD: 61) patients who relapsed during teriflunomide treatment (51.12%) and 73 (SD: 46) who relapsed after switching from teriflunomide to other DMT treatment (7.27%); 86 (SD: 39) patients who recovered after a relapse during teriflunomide treatment (8.57%) and 37 (SD: 38) patients who recovered after a relapse during DMT treatment (3.73%); eventually, 2 (SD: 1) patients dead (0.22%).
The RRMS experienced hypothetical cohort totals, on average, 282 (SD: 145) non-relapsed patients (28.23% of the starting 1000 notional patients), 608 (SD: 111) patients who relapsed during teriflunomide treatment (60.76%); 107 (SD: 51) patients who recovered after a relapse during teriflunomide treatment (10.73%); finally, 3 (SD: 2) patients passed away (0.28%).
Loss of working or leisure time is higher for RRMS naïve patients (205.44 h; range: 169.47–238.61 vs. 181.92 h; range: 156.48–214.06), who, in turn, need less informal care (37.20 h; range: 20.95–57.81 vs. 83.28 h; range: 63.49–105.17).
Cost, LYS, QALYs and incremental cost-utility ratio
Following the societal perspective, after 7 years, the average cost per notional patient is lower for RRMS naïve vs. experienced patients (€108,162.69 vs. €114,945.50) (Table 2).
Healthcare sector costs amount to €89,674.24 and €90,716.92 for RRMS naïve and experienced notional patients (82.91% vs. 78.92% of the overall cost, respectively); out-of-pocket expenses equal €11,461.01 and €17,045.94 for RRMS naïve and experienced notional patients (10.59% vs. 14.83% of the overall cost, respectively), whereas productivity losses and informal care reach €7027.44 and €7182.64 for RRMS naïve and experienced notional patients (6.50% vs. 6.25% of the overall cost, respectively).
For both the hypotetical cohorts, the cost-driver is teriflunomide (59.33% and 67.39% of the overall cost totalized by RRMS naïve and experienced notional patients, respectively).
Despite the similarity in overall mortality (LYS 6.406 vs. 6.402 for RRMS naïve and experienced notional patients, respectively), 7-year QALYs are higher for RRMS naïve patients (3.603 vs. 3.123 per notional patient).
Due to incremental QALYs (+ 0.480 for both perspectives) and cost-savings (healthcare sector perspective: − €1042.68; societal perspective: − €6782.81), teriflunomide is strongly dominant when administered to RRMS naïve vs. experienced notional patients.
Sensitivity analyses
For both healthcare sector and societal viewpoints, OWSA shows that the largest departures from baseline ICUR reported on tornado chart follow from variations in the conditional probability of switching to ocrelizumab due teriflunomide ineffectiveness in RRMS naïve notional patients (from − 1401.30 to + 659.45% vs. base case ICUR) (Figs. 1 and 2).
Results — OWSA — tornado chart — departures from baseline ICUR caused by the most relevant 10 parameters included in OWSA — healthcare sector perspective (€2019). CI, confidence interval; EDSS, Expanded Disability Status Scale; ICUR, incremental cost-utility ratio; LL 95% CI, lower limit 95% CI; OWSA, one-way sensitivity analysis; RRMS, relapsing–remitting multiple sclerosis; UL 95% CI, upper limit 95% CI. Base case ICUR, teriflunomide in RRMS naïve patient is strongly dominant
Results — OWSA — Tornado chart — departures from baseline ICUR caused by the most relevant 10 parameters included in OWSA — societal perspective (€2019). CI, confidence interval; EDSS, Expanded Disability Status Scale; ICUR, incremental cost-utility ratio; LL 95% CI, lower limit 95% CI; OWSA, one-way sensitivity analysis; RRMS, relapsing–remitting multiple sclerosis; UL 95% CI, upper limit 95% CI. Base case ICUR, teriflunomide in RRMS naïve patient is strongly dominant
The conditional probability of switching to fingolimod in RRMS naïve notional patients given poor response to teriflunomide is ranked second (healthcare sector perspective: from − 405.07 to + 307.22%) and third (societal perspective: from − 62.29 to + 47.24%) among the most influential parameters on the base case cost per incremental QALY gained when its sample estimate is replaced by the lower and the upper limits of 95% CI (from − 405.07 to + 307.22%).
Changes in EDSS scores totalled by RRMS-experienced notional patients produce a moderate variation in the baseline ICUR when the healthcare sector standpoint is considered (from − 210.79 to + 37.62%) (Fig. 1).
The mildest impact on the base case ICUR is due to variations in the conditional probability of remission given relapse for both RRMS naïve and experienced notional patients (healthcare sector perspective: from − 77.66 to + 73.43%) and minutes of transportation from RRMS naïve patient’s home to hospital (societal perspective: from − 37.96 to + 25.65%), respectively (Figs. 1 and 2).
Real social discount rate for costs, LYS and QALYs is not ranked among the first 600 (healthcare sector perspective) and 30 (societal perspective) parameters that produce the largest variations in the baseline ICUR.
In the first SSA, ICUR reaches its maximum at year 3 for both healthcare sector (€26,422.27) and societal (€30,661.47) standpoints and then decreases progressively; the strong dominance status for RRMS naïve notional patients is reached at year 7 (Fig. SI2).
The second SSA highlights that reducing adherence probability to 40% increases baseline ICUR of + 1762.31% (health case sector perspective) and + 327.44% (societal perspective) (Table SI53).
Notwithstanding the increased probability of remission after RRMS relapse, the third SSA confirms that teriflunomide in RRMS naïve notional patients is strongly dominant (Table SI54).
As far as PSA results are concerned, CEAC shows higher probability for teriflunomide in RRMS naïve notional patients to be cost-effective as the willingness to pay (WTP) for incremental QALY gained increases. Following the healthcare sector (societal) perspective, the likelihood for teriflunomide in RRMS naïve notional patients to be cost-effective equals 94.52% (95.46%) for a WTP of €25,000 and 95.37% (95.89%) for a WTP of €40,000 (Figs. 3 and 4).
Results — PSA — CEAC and CEAF (1000 out of 1000 threshold values plotted) — healthcare sector perspective (€2019). CEAC, cost-effectiveness acceptability curve; CEAF, cost-effectiveness acceptability frontier; ICUR, incremental cost-utility ratio; NMB, net monetary benefit; PSA, probabilistic sensitivity analysis; RRMS, relapsing–remitting multiple sclerosis; WTP, willingness to pay. Base case ICUR, teriflunomide in RRMS naïve patients is strongly dominant. CEAC for teriflunomide in RRMS naïve patients and CEAF overlap as it is the healthcare programme with the higher probability of being cost-effective and the highest expected NMB CEAF shows that teriflunomide in RRMS naïve patients is the optimal strategy from a threshold value of €0.00 onward (i.e. from A to B)
Results — PSA — CEAC and CEAF (1000 out of 1000 threshold values plotted) — societal perspective (€2019). CEAC, cost-effectiveness acceptability curve; CEAF, cost-effectiveness acceptability frontier; ICUR, incremental cost-utility ratio; NMB, net monetary benefit; PSA, probabilistic sensitivity analysis; RRMS, relapsing–remitting multiple sclerosis; WTP, willingness to pay. Base case ICUR, teriflunomide in RRMS naïve patients is strongly dominant. CEAC for teriflunomide in RRMS naïve patients and CEAF for RRMS naïve patients overlap as it is the healthcare programme with the higher probability of being cost-effective and the highest expected NMB. CEAF shows that teriflunomide in RRMS naïve patients is the optimal strategy from a threshold value of €0.00 onward (i.e. from A to B)
If, being interested in savings only, decision-makers assigned a WTP = 0 per incremental QALY gained, the probability for teriflunomide to be cost-effective in RRMS naïve notional patients would be 52.30% (healthcare sector standpoint) and 79.75% (societal standpoint), respectively.
For both healthcare sector and societal viewpoints, the CEAC of teriflunomide in RRMS naïve patients and CEAF overlap as it is the healthcare programme with the higher probability of being cost-effective and the highest expected NMB from a WTP = 0 onward.
Discussion
This paper reports on methods and results of a 7-year Markov model-supported CUA [3, 13, 14] that compares costs and QALYs totaled by two hypothetical cohorts of RRMS patients on teriflunomide who are, respectively, naïve and experienced vs. previous DMTs.
To the best of our knowledge, our research is innovative in two respects.
First, this is the first comparison of costs and QALYs accrued to RRMS notional patients on teriflunomide who were already or never exposed to DMTs.
Besides, the Markov model-supported CUA was conceived from scratch for the Italian setting instead of being a customization to the local clinical and economic standards of a health economic model originally developed for a different country.
Despite previous researches did not provide consistent recommedations about the cost-effectiveness of teriflunomide [32,33,34,35,36], the basecase analysis and most of the variations made in SAs show that, for both healthcare sector and society standpoints, teriflunomide administered in RRMS naïve rather than experienced patients is cost-saving and, at the same time, produces more QALYs.
OWSA highlights that, for both healthcare sector and societal perspectives, the baseline ICUR is highly sensitive to variations induced in the conditional probability of switching to other DMTs from year 2 onwards given teriflunomide ineffectiveness in RRMS naïve notional patients. This finding is consistent with the remarkable impact of the DMTs (15.57%) on the overall cost totaled by RRMS naïve notional patients in the base case CUA. In addition, since MS experts’ point estimate for this parameter (10.00%) was less optimistic than the mean probability of discontinuation due to ineffectiveness of teriflunomide 7 mg (3.86%) and 14 mg (2.57%) reported in literature (3.86% + 2.57% = 6.43%) [8], the favourable cost-effectiveness profile of teriflunomide in RRMS naïve vs. experienced patients was possibly conservative.
Despite remarkable variations in Markov model key-parameters, such as time horizon, adherence probability to teriflunomide and remission after RRMS relapse, SSAs confirm the baseline results or, at worst, show the base case ICUR not to exceed the upper limit of the informal acceptability range for incremental LYS or QALY gained suggested for the Italian setting (€25,000–€40,000) [29].
PSA shows that there is no evidence that costs of RRMS-experienced patients on teriflunomide may be lower than the ones totalized by their naïve counterparts.
This finding is proved by the intersection of CEACs with the y-axis that represents the one-side p-value for the difference in costs between the two healthcare programmes under comparison, as for WTP = 0 cost containment only is important for decision-makers [14]. These values (52.30% and 79.75% for healthcare sector and societal perspectives, respectively) are higher than the arbitrary 5% one-way p-value.
Conversely, CEACs highlight that the difference in QALYs for RRMS naïve patients on teriflunomide is statistically significant at 5%. This result becomes apparent as, when WTP approaches positive infinity, CEACs tend to 1 minus the (one-tailed) p-value for the difference in QALYs gained by RRMS naïve patients, that is (1–96.52%) = 3.48% for both the adopted viewpoints [14].
More substantively for rationing decisions, CEACs show that, when contrasted against the aforementioned informal acceptability range for incremental LYS or QALY gained recommended for Italy [29], the probability for teriflunomide to be cost-effective in RRMS naïve patients is always higher than 94.50%, regardless of the adopted standpoint. CEAFs confirm CEACs results.
PSA findings can impact Italian MS specialists’ decision-making, whose clinical practice faces tight budget constraints. Prescribing teriflunomide RRMS in naïve patients implies a negligible likelihood of resources misallotment that can be calculated as the difference between 1 and the probability for teriflunomide in RRMS naïve patients to be optimal for each WTP value reported on the x-axis of the CEAF graph. For instance, following the healthcare sector viewpoint, the probability of resources misallocation reaches (1–94.52%) = 5.48% and (1–95.37%) = 4.63% for a WTP of €25,000 and €40,000 [29], respectively.
What are the main limitations of this study?
First, as a head-to-head comparison of teriflunomide in naïve vs. experienced RRMS real patients was unavailable, the parameters needed for populating the Markov model were retrieved from different sources [37]. In addition, a relevant share of the clinical parameters was obtained from the qualified opinion [15] of a convenience sample [16] of 4 MS specialists who coauthored this manuscript. It is also worth noting that, since all the authors gathered together in a meeting aimed at exploring the feasibility of this research, the Delphi panel approach [15] that elicits data from each expert separately was not feasible, as the anonymity requirement would have not been met.
Against the possible criticism that the only robust findings are those obtained from empirical economic evaluation of healthcare programmes piggybacked onto clinical trials [3], it seems worth reminding that healthcare resources allocation based on the results of a Markov model-supported CUA are, in all likelihood, more helpful than decisions made with no support at all [38].
A third limitation, linked to the first one, rests on the evidence that utility values related to different EDSS scores [17] and disutility values due to the adverse events that teriflunomide may cause [20,21,22,23,24] were originally collected for foreign countries. However, the literature claims that, unlike costs, QALYs are less expected to change remakably when utility values are retrieved from researches performed abroad, as, other things being equal, patients sharing the same disease severity tend to report similar utility values [31].
Notwithstanding, it would be interesting to collect empirical utiliy (and disutility) values from a sample of RRMS naïve and experienced patients on teriflunomide in a CUA performed alongside an Italian empirical study [39]. This chance will be particularly welcomed if a validated Italian version of the recent Neuro-QoL utility scoring system is available in the near future [40].
In conclusion, teriflunomide in RRMS naïve vs. experienced patients is cost-effective and possibly strongly dominant from both the healthcare sector and the society viewpoints in Italy.
However, even though decision models are well established in the health economic literature [3, 13, 14], our findings need further confirmation from real-world studies.
Data availability
Not applicable.
Code availability
Not applicable.
References
Battaglia MA, Bezzin D (2017) Estimated prevalence of multiple sclerosis in Italy in 2015. Neurol Sci 38:473–479. https://doi.org/10.1007/s10072-016-2801-9
Trojano M, Bergamaschi R, Amato MP, Comi G, Ghezzi A, Lepore V, Marrosu MG, Mosconi P, Patti F, Ponzio M, Zaratin P, Battaglia MA, Italian Multiple Sclerosis Register Centers Group (2019) The Italian multiple sclerosis register. Neurol Sci 40:155–165. https://doi.org/10.1007/s10072-018-3610-0
Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW (2015) Methods for the economic evaluation of health care programmes, 4th edn. Oxford University Press, Oxford
Zettl UK, Schreiber H, Bauer-Steinhusen U, Glaser T, Hechenbichler K, Hecker M, BETAPATH Study Group (2017) Baseline predictors of persistence to first disease-modifying treatment in multiple sclerosis. Acta Neurol Scand 136:116–121. https://doi.org/10.1111/ane.12705
Paolicelli D, Manni A, Iaffaldano A, Trojano M (2020) Efficacy and safety of oral therapies for relapsing-remitting multiple sclerosis. CNS Drugs 34:65–92. https://doi.org/10.1007/s40263-019-00691-7
Comi G, Radaelli M, Soelberg SP (2017) Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 389:1347–1356. https://doi.org/10.1016/S0140-6736(16)32388-1
O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, Benzerdjeb H, Truffinet P, Wang L, Miller A, Freedman MS, TEMSO Trial Group (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365:1293–1303. https://doi.org/10.1056/NEJMoa1014656
Confavreux C, O’Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, Wolinsky JS, Bagulho T, Delhay JL, Dukovic D, Truffinet P, Kappos L, TOWER Trial Group (2014) Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 13:247–256. https://doi.org/10.1016/S1474-4422(13)70308-9
Miller AE, Wolinsky JS, Kappos L, Comi G, Freedman MS, Olsson TP, Bauer D, Benamor M, Truffinet P, O’Connor PW, TOPIC Study Group (2014) Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 13:977–986. https://doi.org/10.1016/S1474-4422(14)70191-7
Agenzia Italiana del Farmaco. Determina 31 luglio 2014. Regime di rimborsabilita’ e prezzo del medicinale per uso umano «Aubagio» (teriflunomide). (Determina n. 837/2014) (2014) Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, n. 187 del 13 agosto 2014 (Italian)
Liu Z, Liao Q, Wen H, Zhang Y (2021) Disease modifying therapies in relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. Autoimmun Rev 20:102826. https://doi.org/10.1016/j.autrev.2021.102826
Mendes D, Alves C, Batel-Marques F (2016) Benefit-risk of therapies for relapsing-remitting multiple sclerosis: testing the number needed to treat to benefit (NNTB), number needed to treat to harm (NNTH) and the likelihood to be helped or harmed (LHH): a systematic review and meta-analysis. CNS Drugs 30:909–929. https://doi.org/10.1007/s40263-016-0377-9
Sonnenberg FA, Beck JR (1993) Markov models in medical decision making: a practical guide. Med Decis Making 13:322–339. https://doi.org/10.1177/0272989X9301300409
Briggs A, Sculpher M, Claxton K (2006) Decision modelling for health economic evaluation. Oxford University Press, Oxford
O’Hagan A, Buck CE, Daneshkhah A, Eiser JR, Garthwaite PH, Jenkinson DJ, Oakley JE, Rakow T (2006) Uncertain judgements: eliciting experts’ probabilities. Wiley, Chichester
Lohr SL (2010) Sampling: design and analysis, 2nd edn. Brooks/Cole, Boston
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452. https://doi.org/10.1212/wnl.33.11.1444
Iannazzo S, Santoni L, Saleri C, Puma E, Vestri G, Giuliani L, Canonico PL, Centonze D (2016) Analisi di costo-efficacia dell’utilizzo di peginterferone beta-1a nel trattamento della sclerosi multipla recidivante remittente in Italia. Farmeconomia. Health Econ Ther Pathways 17(Suppl 2):13–36. (Italian). https://doi.org/10.7175/fe.v17i2S.1230
Stoppe M, Busch M, Krizek L, Then Bergh F (2017) Outcome of MS relapses in the era of disease-modifying therapy. BMC Neurol 17:151. https://doi.org/10.1186/s12883-017-0927-x
Orme M, Kerrigan J, Tyas D, Russell N, Nixon R (2007) The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health 10:54–60. https://doi.org/10.1111/j.1524-4733.2006.00144.x
Stein JD, Brown GC, Brown MM, Sharma S, Hollands H, Stein HD (2002) The quality of life of patients with hypertension. J Clin Hypertens 4:181–188. https://doi.org/10.1111/j.1524-6175.2002.00970.x
National Institute for Health and Care Excellence (NICE). Teriflunomide for treating relapsing–remitting multiple sclerosis. Technology appraisal guidance. nice.org.uk/guidance/ta303. Accessed 3 Dec 2019
Kolovos S, Bosmans JE, van Dongen JM, van Esveld B, Magai D, van Straten A, van der Feltz-Cornelis C, van Steenbergen-Weijenburg KM, Huijbregts KM, van Marwijk H, Riper H, van Tulder MW (2017) Utility scores for different health states related to depression: individual participant data analysis. Qual Life Res 26:1649–1658. https://doi.org/10.1007/s11136-017-1536-2
Lazzaro C, Barone C, Caprioni F, Cascinu S, Falcone A, Maiello E, Milella M, Pinto C, Reni M, Tortora G (2018) An Italian cost-effectiveness analysis of paclitaxel albumin (nab-paclitaxel) + gemcitabine vs gemcitabine alone for metastatic pancreatic cancer patients: the APICE study. Expert Rev Pharmacoecon Outcomes Res 18:435–446. https://doi.org/10.1080/14737167.2018.1464394
Ministero della Salute. Decreto 8 febbraio 2013. Criteri per la composizione e il funzionamento dei comitati etici. (13A03474) (2013) Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, n. 96 del 24 aprile 2013 (Italian)
Repubblica Italiana. Legge 23 dicembre 1996, n. 662. Misure di razionalizzazione della finanza pubblica (1996) Supplemento ordinario n. 233 alla Gazzetta Ufficiale della Repubblica Italiana, Serie Generale, n. 303 del 28 dicembre 1996 (Italian)
Brouwer W, Rutten F, Koopmanschap M (2001) Costing in economic evaluations. In: Drummond M, McGuire A (eds) Economic evaluation in health care: merging theory with practice. Oxford University Press, Oxford, pp 68–93
Posnett J, Jan S (1996) Indirect cost in economic evaluation: the opportunity cost of unpaid inputs. Health Econ 5:13–23. https://doi.org/10.1002/(SICI)1099-1050(199601)5:1<13::AID-HEC182>3.0.CO;2-J
Fattore G per Gruppo di lavoro Associazione Italiana di Economia Sanitaria (AIES) (2009) Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. PharmacoEconomics – Italian Research Articles 11:83–93 (Italian). https://doi.org/10.1007/BF03320660
Fenwick E, Claxton K, Sculpher M (2001) Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 10:779–787. https://doi.org/10.1002/hec.635
Glick HA, Doshi JA, Sonnad SA, Polsky D (2015) Economic evaluation in clinical trials, 2nd edn. Oxford University Press, Oxford
Couto E, Hamidi V, Ringerike T, Odgaard-Jensen J, Harboe I, Klemp M. Medicines used for multiple sclerosis – a health technology assessment. https://www.ncbi.nlm.nih.gov/books/NBK482112/pdf/Bookshelf_NBK482112.pdf. Accessed 26 Nov 2019
Boster A, Nicholas J, Wu N, Yeh WS, Fay M, Edwards M, Huang MY, Lee A (2017) Comparative effectiveness research of disease-modifying therapies for the management of multiple sclerosis: analysis of a large health insurance claims database. Neurol Ther 6:91–102. https://doi.org/10.1007/s40120-017-0064-x
Bowen JD, Kozma CM, Grosso MM, Phillips AL (2018) A real-world comparison of relapse rates, healthcare costs and resource use among patients with multiple sclerosis newly initiating subcutaneous interferon beta-1a versus oral disease-modifying drugs. Mult Scler J Exp Transl Clin 4:2055217318819031. https://doi.org/10.1177/2055217318819031
Zimmermann M, Brouwer E, Tice JA, Seidner M, Loos AM, Liu S, Chapman RH, Kumar V, Carlson JJ (2018) Disease-modifying therapies for relapsing-remitting and primary progressive multiple sclerosis: a cost-utility analysis. CNS Drugs 32:1145–1157. https://doi.org/10.1007/s40263-018-0566-9
Xu Y, Mao N, Chirikov V, Du F, Yeh YC, Liu L, Liu R, Gao X (2019) Cost-effectiveness of teriflunomide compared to interferon beta1b for relapsing multiple sclerosis patients in China. Clin Drug Investig 39:331–340. https://doi.org/10.1007/s40261-019-00750-3
Caro JJ, Briggs AH, Siebert U, Kuntz KM, ISPOR-SMDM Modeling Good Research Practices Task Force (2012) Modeling good research practices – overview: a report of the ISPOR SMDM Modeling Good Research Practices Task Force-1. Value Health 15:796–803. https://doi.org/10.1016/j.jval.2012.06.012
Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR, ISPOR Task Force on Good Research Practices--Modeling Studies (2003) Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices-Modeling Studies. Value Health 6:9–17. https://doi.org/10.1046/j.1524-4733.2003.00234.x
Mozzi A, Meregaglia M, Lazzaro C, Tornatore V, Belfiglio M, Fattore G (2016) A comparison of EQ-5D health-related utilities using Italian, UK, and US preference weights in a patients sample. Clinicoecon Outcomes Res 8:267–274. https://doi.org/10.2147/CEOR.S98226
Matza LS, Phillips G, Dewitt B, Stewart KD, Cella D, Feeny D, Hanmer J, Miller DM, Revicki DA (2020) A scoring algorithm for deriving utility values from the neuro-QoL for patients with multiple sclerosis. Med Decis Making 40:897–911. https://doi.org/10.1177/0272989X20951782
Funding
This research was made possible; thanks to an unrestricted grant from Sanofi S.r.l, which has had no influence of the interpretation of data and the final conclusions drawn.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
During the past 3 years, CL has received research grants, speaker or consultancy fees from AstraZeneca S.p.A., Boehringer Ingelheim Italia S.p.A., CSL Behring S.p.A., Ferring S.p.A., Ipsen S.p.A., Roche S.p.A., Sanofi S.r.l., Santen GmbH, Shire S.p.A, Sobi S.p.A.
RB has served on scientific advisory boards for Biogen, Merck-Serono, Novartis, Sanofi-Genzyme; received research support from Almirall, Bayer, Biogen, Merck-Serono, Novartis, Sanofi-Genzyme; received support for travel and congress from Biogen, Roche, Merck-Serono, Sanofi-Genzyme, Teva; received honoraria for speaking engagement from Biogen, Merck-Serono, Novartis, Sanofi-Genzyme.
MZ has served on scientific advisory boards and received honoraria for speaking or support for travel and congress attendance from Almirall, Biogen, Merck-Serono, Novartis, Sanofi-Genzyme.
During the past 3 years, RT has received speaker or consultancy fees from Biogen, Merck-Serono, Novartis, Roche, Sanofi and Teva.
DP has received honoraria for consultancy and/or speaking from Biogen Idec, Merck-Serono, Bayer-Schering, Sanofi-Aventis, TEVA, Novartis and Genzyme.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 705 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lazzaro, C., Bergamaschi, R., Zaffaroni, M. et al. Cost-utility analysis of teriflunomide in naïve vs. previously treated patients with relapsing–remitting multiple sclerosis in Italy. Neurol Sci 43, 4933–4944 (2022). https://doi.org/10.1007/s10072-022-06022-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06022-x