Abstract
Purpose
Some elders with subjective cognitive deficits (SCD) develop prodromal phase of dementia over time; however, little is known about how they differ from those with normal cognition (NC). Thus, we aim to distinguish the differences in the brain network of elders with SCD and NC.
Methods
Multiple diffusion-weighted images (DWI) and T1-weighted images were obtained from 18 subjects with NC and 26 subjects with SCD. Using network-based statistics (NBS) analysis, we extracted abnormal brain subnetworks and localized abnormal brain connectivity. We also ran correlation analysis to compare the affected regions and the results of the neurocognitive assessments.
Results
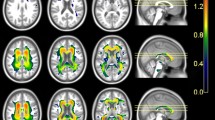
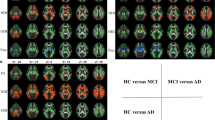
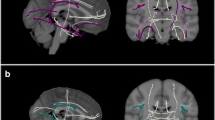
Altered subnetworks were found in the superior parietal gyrus, angular gyrus, precuneus, posterior cingulum, putamen, precentral gyrus, postcentral gyrus, and paracentral lobule. They were also associated with scores on the word list recall, word list recognition, and Boston naming test.
Conclusions
Elders with SCD had distinctive brain network alterations when compared with those of elders with NC. The results are also in line with the previously identified characteristics of mild cognitive impairment (MCI) and of Alzheimer’s disease (AD) in a milder form. We speculate that it may be possible to predict AD progression early in the SCD stage using NBS analysis.


Similar content being viewed by others
References
Rodda J, Dannhauser T, Cutinha DJ, Shergill SS, Walker Z (2011) Subjective cognitive impairment: functional MRI during a divided attention task. Eur Psychiatry 26(7):457–462. https://doi.org/10.1016/j.eurpsy.2010.07.003
Hafkemeijer A, Altmann-Schneider I, Oleksik AM, van de Wiel L, Middelkoop HA, van Buchem MA, van der Grond J, Rombouts SA (2013) Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect 3(4):353–362. https://doi.org/10.1089/brain.2013.0144
Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, Suliman H, Wagner M, Schild HH, Jessen F (2010) Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement Geriatr Cogn Disord 29(1):75–81. https://doi.org/10.1159/000264630
Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18(4):351–357
Lopez-Sanz D, Garces P, Alvarez B, Delgado-Losada ML, Lopez-Higes R, Maestu F (2017) Network disruption in the preclinical stages of Alzheimer’s disease: from subjective cognitive decline to mild cognitive impairment. Int J Neural Syst 27:1750041. https://doi.org/10.1142/s0129065717500411:1750041
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM et al (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10(6):844–852. https://doi.org/10.1016/j.jalz.2014.01.001
Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Trollor JN, Draper B, Sachdev PS (2010) Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry 18(8):701–710
van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, Mutsaers ER, Bollen EL, Admiraal-Behloul F, Westendorp RG, Middelkoop HA (2004) Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol 251(6):671–675. https://doi.org/10.1007/s00415-004-0390-7
Striepens N, Scheef L, Wind A, Meiberth D, Popp J, Spottke A, Kolsch H, Wagner M, Jessen F (2011) Interaction effects of subjective memory impairment and ApoE4 genotype on episodic memory and hippocampal volume. Psychol Med 41(9):1997–2006. https://doi.org/10.1017/s0033291711000067
Jessen F (2014) Subjective and objective cognitive decline at the pre-dementia stage of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 264(Suppl 1):S3–S7. https://doi.org/10.1007/s00406-014-0539-z
Jenkins A, Tales A, Tree J, Bayer A (2015) Are we ready? The construct of subjective cognitive impairment and its utilization in clinical practice: a preliminary UK-based service evaluation. J Alzheimers Dis 48(Suppl 1):S25–S31. https://doi.org/10.3233/jad-150541
Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Schild HH, Scheef L (2006) Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging 27(12):1751–1756. https://doi.org/10.1016/j.neurobiolaging.2005.10.010
Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F (2011) Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry 68(8):845–852. https://doi.org/10.1001/archgenpsychiatry.2011.80
Yue L, Wang T, Wang J, Li G, Wang J, Li X, Li W, Hu M, Xiao S (2018) Asymmetry of Hippocampus and amygdala defect in subjective cognitive decline among the community dwelling Chinese. Front Psychiatry 9:226. https://doi.org/10.3389/fpsyt.2018.00226
Shu N, Wang X, Bi Q, Zhao T, Han Y (2018) Disrupted topologic efficiency of white matter structural connectome in individuals with subjective cognitive decline. Radiology 286(1):229–238. https://doi.org/10.1148/radiol.2017162696
Wang XN, Zeng Y, Chen GQ, Zhang YH, Li XY, Hao XY, Yu Y, Zhang M, Sheng C, Li YX, Sun Y, Li HY, Song Y, Li KC, Yan TY, Tang XY, Han Y (2016) Abnormal organization of white matter networks in patients with subjective cognitive decline and mild cognitive impairment. Oncotarget 7(31):48953–48962. https://doi.org/10.18632/oncotarget.10601
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198. https://doi.org/10.1038/nrn2575
Sporns O (2010) Networks of the brain. MIT Press, Cambridge
Zalesky A, Fornito A, Bullmore ET (2010) Network-based statistic: identifying differences in brain networks. Neuroimage 53(4):1197–1207. https://doi.org/10.1016/j.neuroimage.2010.06.041
Filippi M, Basaia S, Canu E, Imperiale F, Meani A, Caso F, Magnani G, Falautano M, Comi G, Falini A, Agosta F (2017) Brain network connectivity differs in early-onset neurodegenerative dementia. Neurology 89(17):1764–1772. https://doi.org/10.1212/wnl.0000000000004577
Worsley KJ, Evans AC, Marrett S, Neelin P (1992) A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12(6):900–918. https://doi.org/10.1038/jcbfm.1992.127
Mori S, Barker PB (1999) Diffusion magnetic resonance imaging: its principle and applications. Anat Rec 257(3):102–109
Wang R, Benner T, Sorensen A, Wedeen VJ (2007) Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med 15:3720
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20(1):45–57. https://doi.org/10.1109/42.906424
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1):273–289. https://doi.org/10.1006/nimg.2001.0978
de Reus MA, van den Heuvel MP (2013) Estimating false positives and negatives in brain networks. Neuroimage 70:402–409. https://doi.org/10.1016/j.neuroimage.2012.12.066
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–1069. https://doi.org/10.1016/j.neuroimage.2009.10.003
Watts DJ, Strogatz SH (1998) Collective dynamics of ‘small-world’ networks. Nature 393(6684):440–442. https://doi.org/10.1038/30918
Guimerà R, Nunes Amaral LA (2005) Functional cartography of complex metabolic networks. Nature 433(7028):895–900. https://doi.org/10.1038/nature03288
Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, Egan GF, Pantelis C (2011) Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry 69(1):80–89. https://doi.org/10.1016/j.biopsych.2010.08.022
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4):1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024
Myung W, Han CE, Fava M, Mischoulon D, Papakostas GI, Heo JY, Kim KW, Kim ST, Kim DJ, Kim DK et al (2016) Reduced frontal-subcortical white matter connectivity in association with suicidal ideation in major depressive disorder. Transl Psychiatry 6(6):e835. https://doi.org/10.1038/tp.2016.110
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 57(1):289–300
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97(20):11050–11055. https://doi.org/10.1073/pnas.200033797
Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101:4637–4642. https://doi.org/10.1073/pnas.0308627101
Wang Y, Risacher SL, West JD, McDonald BC, Magee TR, Farlow MR, Gao S, O'Neill DP, Saykin AJ (2014) Altered default mode network connectivity in older adults with cognitive complaints and amnestic mild cognitive impairment. J Alzheimers Dis 35:751–760. https://doi.org/10.3233/jad-130080
Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T et al (2010) Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 63:178–188. https://doi.org/10.1016/j.neuron.2009.07.003
Wang J, Zuo X, Dai Z, Xia M, Zhao Z, Zhao X, Jia J, Han Y, He Y (2013) Disrupted functional brain connectome in individuals at risk for Alzheimer’s disease. Biol Psychiatry 73:472–481. https://doi.org/10.1016/j.biopsych.2012.03.026
de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RGJ, Bollen ELEM, de Bruin PW, Middelkoop HAM, van Buchem MA, van der Grond J (2008) Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study. Brain. 131:3277–3285. https://doi.org/10.1093/brain/awn278
Yao H, Liu Y, Zhou B, Zhang Z, An N, Wang P, Wang L, Zhang X, Jiang T (2013) Decreased functional connectivity of the amygdala in Alzheimer’s disease revealed by resting-state fMRI. Eur J Radiol 82:1531–1538. https://doi.org/10.1016/j.ejrad.2013.03.019
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259
Huang S-Y, Hsu J-L, Lin K-J, Liu H-L, Wey S-P, Hsiao I-T, Alzheimer’s Disease Neuroimaging I (2018) Characteristic patterns of inter- and intra-hemispheric metabolic connectivity in patients with stable and progressive mild cognitive impairment and Alzheimer’s disease. SC Reports. https://doi.org/10.1038/s41598-018-31794-8
Alegret M, Cuberas-Borros G, Espinosa A, Valero S, Hernandez I, Ruiz A, Becker JT, Rosende-Roca M, Mauleon A, Sotolongo O et al (2014) Cognitive, genetic, and brain perfusion factors associated with four year incidence of Alzheimer’s disease from mild cognitive impairment. J Alzheimers Dis 41(3):739–748. https://doi.org/10.3233/jad-132516
Karavasilis E, Parthimos TP, Papatriantafyllou JD, Papageorgiou SG, Kapsas G, Papanicolaou AC, Seimenis I (2017) A specific pattern of gray matter atrophy in Alzheimer’s disease with depression. https://doi.org/10.1007/s00415-017-8603-z
Leto L, Feola M (2014) Cognitive impairment in heart failure patients. J Geriatr Cardiol 11(4):316–328. https://doi.org/10.11909/j.issn.1671-5411.2014.04.007
Achard SBE (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3:e17
Seghier ML (2012) The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19:43–61. https://doi.org/10.1177/1073858412440596
Bonnici HM, Richter FR, Yazar Y, Simons JS (2016) Multimodal feature integration in the angular gyrus during episodic and semantic retrieval. J Neurosci 36:5462–5471. https://doi.org/10.1523/jneurosci.4310-15.2016
Xie C, Bai F, Yu H, Shi Y, Yuan Y, Chen G, Li W, Chen G, Zhang Z, Li S-J (2015) Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. Neuroimage. 63:320–327. https://doi.org/10.1016/j.neuroimage.2012.06.062
Jacobs HI, Van Boxtel MP, Jolles J, Verhey FR, Uylings HB (2012) Parietal cortex matters in Alzheimer’s disease: an overview of structural, functional and metabolic findings. Neurosci Biobehav Rev 36(1):297–309. https://doi.org/10.1016/j.neubiorev.2011.06.009
Koenigs M, Barbey AK, Postle BR, Grafman J (2009) Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci 29(47):14980–14986. https://doi.org/10.1523/jneurosci.3706-09.2009
Ng VW, Bullmore ET, de Zubicaray GI, Cooper A, Suckling J, Williams SC (2001) Identifying rate-limiting nodes in large-scale cortical networks for visuospatial processing: an illustration using fMRI. J Cogn Neurosci 13(4):537–545
Hodges JR, Salmon DP, Butters N (1991) The nature of the naming deficit in Alzheimer’s and Huntington’s disease. Brain. 114:1547–1558. https://doi.org/10.1093/brain/114.4.1547
Flanagan KJ, Copland DA, Chenery HJ, Byrne GJ, Angwin AJ (2013) Alzheimer’s disease is associated with distinctive semantic feature loss. Neuropsychologia. 51:2016–2025. https://doi.org/10.1016/j.neuropsychologia.2013.06.008
Delbeuck X, Van der Linden M, Collette F (2003) Alzheimer’s disease as a disconnection syndrome. Neuropsychol Rev 13(2):79–92
Vecchio F, Miraglia F, Piludu F, Granata G, Romanello R, Caulo M, Onofrj V, Bramanti P, Colosimo C, Rossini PM (2017) “Small world” architecture in brain connectivity and hippocampal volume in Alzheimer’s disease: a study via graph theory from EEG data. Brain Imaging Behav 11(2):473–485. https://doi.org/10.1007/s11682-016-9528-3
Huang J, Friedland RP, Auchus AP (2007) Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am J Neuroradiol 28(10):1943–1948. https://doi.org/10.3174/ajnr.A0700
Selnes P, Fjell AM, Gjerstad L, Bjornerud A, Wallin A, Due-Tonnessen P, Grambaite R, Stenset V, Fladby T (2012) White matter imaging changes in subjective and mild cognitive impairment. Alzheimers Dement 8(5 Suppl):S112–S121. https://doi.org/10.1016/j.jalz.2011.07.001
Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34(1):144–155. https://doi.org/10.1016/j.neuroimage.2006.09.018
Aganj I, Lenglet C, Jahanshad N, Yacoub E, Harel N, Thompson PM, Sapiro G (2011) A Hough transform global probabilistic approach to multiple-subject diffusion MRI tractography. Med Image Anal 15(4):414–425. https://doi.org/10.1016/j.media.2011.01.003
Funding
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) that was funded by the Ministry of Health & Welfare, Republic of Korea (HG.J., HC15C1509, HG.J and CE.H., HI19C0645); the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (HG.J., NRF-2015R1C1A1A01052172); and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education of the Government of the Republic of Korea (CE.H., 2016R1D1A1B03934990).
Author information
Authors and Affiliations
Contributions
HG. Jeong and CE. Han designed the study and supervised the data collection, data analysis, and writing. S. Lee and H. Youn collected the neuroimaging and neurocognitive data and wrote the article. D. Kim and M. Choi carried out the data analysis and wrote the article. Lastly, S. Suh was responsible for data acquisition, subject selection, and quality controls.
Corresponding authors
Ethics declarations
Statement of ethics
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, D., Lee, S., Choi, M. et al. Diffusion tensor imaging reveals abnormal brain networks in elderly subjects with subjective cognitive deficits. Neurol Sci 40, 2333–2342 (2019). https://doi.org/10.1007/s10072-019-03981-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-03981-6