Abstract
Objective
Although olfaction dysfunction is now considered as an established clinical marker of prodromal Parkinson disease (PD), little is known about the neural underpinnings of olfaction dysfunction in the prodromal phase of PD. The aim of this study was to examine the microstructural association of olfaction in prodromal PD compared to early stage drug-naïve PD patients.
Methods
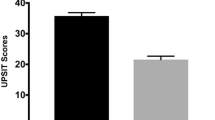
Diffusion MRI connectometry was conducted on 18 early PD and 17 prodromal PD patients to investigate the differences in group in terms of altered connectivity, i.e., integrity of white matter tracts, and subsequently to study the correlation of University of Pennsylvania Smell Identification Test (UPSIT) score to white matter integrity in each group using a multiple regression model considering age, sex, RBD, and MoCA, as covariates.
Results
Individuals with prodromal PD had significantly higher quantitative anisotropy (QA) comparing with PD patients in bilateral middle cerebellar peduncles and right arcuate fasciculus. Multiple regression analysis in prodromal PD demonstrated positive association between UPSIT score and connectivity in left and right subgenual cingulum, right inferior fronto-occipital fasciculus, left corticospinal tract, left parietopontine, left corticothalamic tract, and the body and the splenium of corpus callosum.
Conclusion
These results indicate that PD and prodromal PD patients, which were matched for sex, UPSIT, and MoCA scores, have different white matter fiber architecture. Thus, it is postulated that olfaction dysfunction in prodromal and early clinical phases of PD may involve distinct pathogenesis. Increased network connectivity in prodromal and early PD may suggest the neural compensation.

Similar content being viewed by others
References
Brooks DJ (1998) The early diagnosis of Parkinson’s disease. Ann Neurol 44(3 Suppl 1):S10–S18
Ghazi Sherbaf F, Rostam Abadi Y, Mojtahed Zadeh M, Ashraf-Ganjouei A, Sanjari Moghaddam H, Aarabi MH (2018) Microstructural changes in patients with Parkinson’s disease comorbid with REM sleep behaviour disorder and depressive symptoms. Front Neurol 9:441. https://doi.org/10.3389/fneur.2018.00441
Ansari M, Adib Moradi S, Ghazi Sherbaf F, Hedayatnia A, Aarabi MH (2018) Comparison of structural connectivity in Parkinson’s disease with depressive symptoms versus non-depressed: a diffusion MRI connectometry study. Int Psychogeriatr:1–8. https://doi.org/10.1017/s1041610218000170
Ashraf-Ganjouei A, Majd A, Javinani A, Aarabi MH (2018) Autonomic dysfunction and white matter microstructural changes in drug-naïve patients with Parkinson’s disease. PeerJ 6:e5539. https://doi.org/10.7717/peerj.5539
Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G (2015) MDS research criteria for prodromal Parkinson’s disease. Mov Dis 30(12):1600–1611. https://doi.org/10.1002/mds.26431
Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, Goetz CG, Halliday GM, Hardy J, Lang AE, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G (2014) Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Dis 29(4):454–462. https://doi.org/10.1002/mds.25844
Salat D, Noyce AJ, Schrag A, Tolosa E (2016) Challenges of modifying disease progression in prediagnostic Parkinson’s disease. Lancet Neurol 15(6):637–648. https://doi.org/10.1016/S1474-4422(16)00060-0
Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, Ziemssen T (2012) Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord 27(5):617–626
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Chu S, Downes JJ (2000) Odour-evoked autobiographical memories: psychological investigations of Proustian phenomena. Chem Senses 25(1):111–116
Doty RL (2012) Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 8(6):329–339. https://doi.org/10.1038/nrneurol.2012.80
Berg D, Marek K, Ross GW, Poewe W (2012) Defining at-risk populations for Parkinson’s disease: lessons from ongoing studies. Mov Disord 27(5):656–665
Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H (2007) Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov Disord 22(6):839–842
Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR (2008) Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol 63(2):167–173
Ponsen MM, Stoffers D, Twisk JW, Wolters EC, Berendse HW (2009) Hyposmia and executive dysfunction as predictors of future Parkinson’s disease: a prospective study. Mov Disord 24(7):1060–1065
Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY (2011) Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol 69(5):811–818
Mahlknecht P, Iranzo A, Högl B, Frauscher B, Müller C, Santamaría J, Tolosa E, Serradell M, Mitterling T, Gschliesser V (2015) Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology 84(7):654–658
Jennings D, Stern M, Siderowf A, Eberly S, Oakes D, Marek K (2015) Longitudinal imaging and phenoconversion in the PARS prodromal cohort. Mov Disord 30:S383
Postuma RB, Berg D (2016) Advances in markers of prodromal Parkinson disease. Nat Rev Neurol 12(11):622–634
Rodríguez-violante M, Ospina-García N, Pérez-Lohman C, Cervantes-Arriaga A (2017) Spotlight on olfactory dysfunction in Parkinson’s disease. J Parkinsonism Restless Legs Syndr 55:33–41. https://doi.org/10.2147/JPRLS.S125390
Haehner A, Boesveldt S, Berendse HW, Mackay-Sim A, Fleischmann J, Silburn PA, Johnston AN, Mellick GD, Herting B, Reichmann H, Hummel T (2009) Prevalence of smell loss in Parkinson’s disease--a multicenter study. Parkinsonism Relat Disord 15(7):490–494. https://doi.org/10.1016/j.parkreldis.2008.12.005
Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW (2004) Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol 56(2):173–181. https://doi.org/10.1002/ana.20160
Stern MB, Doty RL, Dotti M, Corcoran P, Crawford D, McKeown DA, Adler C, Gollomp S, Hurtig H (1994) Olfactory function in Parkinson’s disease subtypes. Neurology 44(2):266–268
Doty RL, Shaman P, Dann M (1984) Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 32(3):489–502
Baba T, Kikuchi A, Hirayama K, Nishio Y, Hosokai Y, Kanno S, Hasegawa T, Sugeno N, Konno M, Suzuki K, Takahashi S, Fukuda H, Aoki M, Itoyama Y, Mori E, Takeda A (2012) Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: a 3 year longitudinal study. Brain 135(Pt 1):161–169. https://doi.org/10.1093/brain/awr321
Baba T, Takeda A, Kikuchi A, Nishio Y, Hosokai Y, Hirayama K, Hasegawa T, Sugeno N, Suzuki K, Mori E, Takahashi S, Fukuda H, Itoyama Y (2011) Association of olfactory dysfunction and brain. Metabolism in Parkinson’s disease. Mov Dis 26(4):621–628. https://doi.org/10.1002/mds.23602
Berendse HW, Booij J, Francot CM, Bergmans PL, Hijman R, Stoof JC, Wolters EC (2001) Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol 50(1):34–41
Bohnen NI, Gedela S, Herath P, Constantine GM, Moore RY (2008) Selective hyposmia in Parkinson disease: association with hippocampal dopamine activity. Neurosci Lett 447(1):12–16. https://doi.org/10.1016/j.neulet.2008.09.070
Bohnen NI, Gedela S, Kuwabara H, Constantine GM, Mathis CA, Studenski SA, Moore RY (2007) Selective hyposmia and nigrostriatal dopaminergic denervation in Parkinson’s disease. J Neurol 254(1):84–90. https://doi.org/10.1007/s00415-006-0284-y
Bohnen NI, Muller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, Frey KA (2010) Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain 133(Pt 6):1747–1754. https://doi.org/10.1093/brain/awq079
Siderowf A, Newberg A, Chou KL, Lloyd M, Colcher A, Hurtig HI, Stern MB, Doty RL, Mozley PD, Wintering N, Duda JE, Weintraub D, Moberg PJ (2005) [99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology 64(10):1716–1720. https://doi.org/10.1212/01.wnl.0000161874.52302.5d
Su M, Wang S, Fang W, Zhu Y, Li R, Sheng K, Zou D, Han Y, Wang X, Cheng O (2015) Alterations in the limbic/paralimbic cortices of Parkinson’s disease patients with hyposmia under resting-state functional MRI by regional homogeneity and functional connectivity analysis. Parkinsonism Relat Disord 21(7):698–703. https://doi.org/10.1016/j.parkreldis.2015.04.006
Yeh F-C, Tang P-F, Tseng W-YI (2013) Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. NeuroImage: Clinical 2:912–921. https://doi.org/10.1016/j.nicl.2013.06.014
Leemans A, Jeurissen B, Sijbers J, Jones D (2009) ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. In: 17th Annual Meeting of Intl Soc Mag Reson Med. p 3537
Ghazi Sherbaf F, Mojtahed Zadeh M, Haghshomar M, Aarabi MH (2018) Posterior limb of the internal capsule predicts poor quality of life in patients with Parkinson’s disease: connectometry approach. Acta Neurol Belg. https://doi.org/10.1007/s13760-018-0910-3
Ghazi Sherbaf F, Same K, Aarabi MH (2018) High angular resolution diffusion imaging correlates of depression in Parkinson’s disease: a connectometry study. Acta Neurol Belg. https://doi.org/10.1007/s13760-018-0937-5
Mojtahed Zadeh M, Ashraf-Ganjouei A, Ghazi Sherbaf F, Haghshomar M, Aarabi MH (2018) White matter tract alterations in drug-naive Parkinson’s disease patients with impulse control disorders. Front Neurol 9:163. https://doi.org/10.3389/fneur.2018.00163
Lee EY, Eslinger PJ, Du G, Kong L, Lewis MM, Huang X (2014) Olfactory-related cortical atrophy is associated with olfactory dysfunction in Parkinson’s disease. Mov Dis 29(9):1205–1208. https://doi.org/10.1002/mds.25829
van Uem JM, Marinus J, Canning C, van Lummel R, Dodel R, Liepelt-Scarfone I, Berg D, Morris ME, Maetzler W (2016) Health-related quality of life in patients with Parkinson’s disease—a systematic review based on the ICF model. Neurosci Biobehav Rev 61:26–34
Wattendorf E, Welge-Lussen A, Fiedler K, Bilecen D, Wolfensberger M, Fuhr P, Hummel T, Westermann B (2009) Olfactory impairment predicts brain atrophy in Parkinson’s disease. J Neurosci 29(49):15410–15413. https://doi.org/10.1523/jneurosci.1909-09.2009
Kwon HG, Hong JH, Jang SH (2011) Anatomic location and somatotopic arrangement of the corticospinal tract at the cerebral peduncle in the human brain. AJNR Am J Neuroradiol 32(11):2116–2119. https://doi.org/10.3174/ajnr.A2660
Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, Coffey C, Kieburtz K, Flagg E, Chowdhury S (2011) The parkinson progression marker initiative (PPMI). Prog Neurobiol 95(4):629–635
Yeh FC, Tseng WY (2011) NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. NeuroImage 58(1):91–99. https://doi.org/10.1016/j.neuroimage.2011.06.021
Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI (2013) Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 8(11):e80713. https://doi.org/10.1371/journal.pone.0080713
Schmahmann JD, Smith EE, Eichler FS, Filley CM (2008) Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 1142:266–309. https://doi.org/10.1196/annals.1444.017
Tessa C, Lucetti C, Giannelli M, Diciotti S, Poletti M, Danti S, Baldacci F, Vignali C, Bonuccelli U, Mascalchi M, Toschi N (2014) Progression of brain atrophy in the early stages of Parkinson’s disease: a longitudinal tensor-based morphometry study in de novo patients without cognitive impairment. Hum Brain Mapp 35(8):3932–3944. https://doi.org/10.1002/hbm.22449
Ansari M, Rahmani F, Dolatshahi M, Pooyan A, Aarabi MH (2017) Brain pathway differences between Parkinson’s disease patients with and without REM sleep behavior disorder. Sleep Breath 21(1):155–161. https://doi.org/10.1007/s11325-016-1435-8
Hall JM, Ehgoetz Martens KA, Walton CC, O'Callaghan C, Keller PE, Lewis SJ, Moustafa AA (2016) Diffusion alterations associated with Parkinson’s disease symptomatology: a review of the literature. Parkinsonism Relat Disord 33:12–26. https://doi.org/10.1016/j.parkreldis.2016.09.026
Sobhani S, Rahmani F, Aarabi MH, Sadr AV (2017) Exploring white matter microstructure and olfaction dysfunction in early parkinson disease: diffusion MRI reveals new insight. Brain Imaging Behav. https://doi.org/10.1007/s11682-017-9781-0
Wen MC, Heng HSE, Hsu JL, Xu Z, Liew GM, Au WL, Chan LL, Tan LCS, Tan EK (2017) Structural connectome alterations in prodromal and de novo Parkinson’s disease patients. Parkinsonism Relat Disord 45:21–27. https://doi.org/10.1016/j.parkreldis.2017.09.019
Nigro S, Riccelli R, Passamonti L, Arabia G, Morelli M, Nistico R, Novellino F, Salsone M, Barbagallo G, Quattrone A (2016) Characterizing structural neural networks in de novo Parkinson disease patients using diffusion tensor imaging. Hum Brain Mapp 37(12):4500–4510. https://doi.org/10.1002/hbm.23324
Wei L, Zhang J, Long Z, Wu GR, Hu X, Zhang Y, Wang J (2014) Reduced topological efficiency in cortical-basal Ganglia motor network of Parkinson’s disease: a resting state fMRI study. PLoS One 9(10):e108124. https://doi.org/10.1371/journal.pone.0108124
Scherfler C, Frauscher B, Schocke M, Iranzo A, Gschliesser V, Seppi K, Santamaria J, Tolosa E, Hogl B, Poewe W (2011) White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study. Ann Neurol 69(2):400–407. https://doi.org/10.1002/ana.22245
Shao Y, Wang L, Ye E, Jin X, Ni W, Yang Y, Wen B, Hu D, Yang Z (2013) Decreased thalamocortical functional connectivity after 36 hours of total sleep deprivation: evidence from resting state FMRI. PLoS One 8(10):e78830. https://doi.org/10.1371/journal.pone.0078830
Welge-Lussen A, Wattendorf E, Schwerdtfeger U, Fuhr P, Bilecen D, Hummel T, Westermann B (2009) Olfactory-induced brain activity in Parkinson’s disease relates to the expression of event-related potentials: a functional magnetic resonance imaging study. Neuroscience 162(2):537–543. https://doi.org/10.1016/j.neuroscience.2009.04.050
Gottfried JA, Zald DH (2005) On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Res Brain Res Rev 50(2):287–304. https://doi.org/10.1016/j.brainresrev.2005.08.004
Ibarretxe-Bilbao N, Junque C, Marti MJ, Valldeoriola F, Vendrell P, Bargallo N, Zarei M, Tolosa E (2010) Olfactory impairment in Parkinson’s disease and white matter abnormalities in central olfactory areas: a voxel-based diffusion tensor imaging study. Mov Dis 25(12):1888–1894. https://doi.org/10.1002/mds.23208
Wen M-C, Xu Z, Lu Z, Chan LL, Tan EK, Tan LCS (2017) Microstructural network alterations of olfactory dysfunction in newly diagnosed Parkinson’s disease. Sci Rep 7(1):12559. https://doi.org/10.1038/s41598-017-12947-7
Sunwoo MK, Cha J, Ham JH, Song SK, Hong JY, Lee JM, Sohn YH, Lee PH (2015) Olfactory performance and resting state functional connectivity in non-demented drug naive patients with Parkinson’s disease. Hum Brain Mapp 36(5):1716–1727. https://doi.org/10.1002/hbm.22732
Acknowledgments
We thank Christian Beckmann and Simon Eickhoff for their advice on data analysis. Data used in this article were obtained from the Parkinsons Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
Funding
This dataset of this work was funded by grants from the Michael J Fox Foundation for Parkinson’s Research, the W Garfield Weston Foundation, and the Alzheimer’s Association, the Canadian Institutes for Health Research, and the Natural Sciences and Engineering Research Council of Canada. PPMI is sponsored and partially funded by the Michael J Fox Foundation for Parkinsons Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline (GSK), Eli Lilly and Company, Lundbeck, Merck, Meso Scale Discovery (MSD), Pfizer, Piramal Imaging, Roche, Servier, and UCB (www.ppmi-info.org/fundingpartners).
Author information
Authors and Affiliations
Contributions
H.S.M, M.H.A. contributed to the conception and design of the study; M.H.A. contributed to data collection and analysis; and H.S.M, M.D, and E.S.D contributed to writing and revising the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed here, including human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sanjari Moghaddam, H., Dolatshahi, M., Salardini, E. et al. Association of olfaction dysfunction with brain microstructure in prodromal Parkinson disease. Neurol Sci 40, 283–291 (2019). https://doi.org/10.1007/s10072-018-3629-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3629-2