Abstract
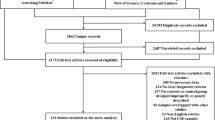
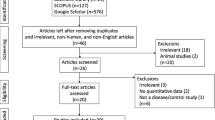
As a biomarker of axonal injury, neurofilament light chain (NFL) in multiple system atrophy (MSA) patients and Parkinson’s disease (PD) patients has been investigated by numerous studies. However, cerebrospinal fluid (CSF) NFL changes are conflicting in MSA patients relative to PD patients to date. Therefore, the current study was carried out to find out possible heterogeneity sources. Furthermore, “Neurofilament”, “Neurofilament light chain” and “Multiple system atrophy” were employed to search “PubMed”, “Springer” and “Medline” databases until August 2016 with standard mean difference (Std.MD) being calculated. In addition, subgroup analysis and meta-regression were performed to assess possible heterogeneity sources. Nine studies were pooled, in which 212 MSA patients and 373 PD patients were involved. Moreover, CSF NFL in MSA patients was higher than that in PD patients [pooled Std.MD = 1.56, 95% CI (1.12, 2.00), p < 0.00001] with significant heterogeneity (I 2 = 76%). Besides, population variations, sample size, the difference in CSF phosphorylated tau (p-tau) levels between MSA patients and PD patients, and Hoehn–Yahr staging of PD patients were the main heterogeneity sources. As shown by meta-regression, Hedges’s g of CSF NFL was correlated with CSF Std.MD of α-synuclein between MSA patients and healthy controls (r = −1.34824, p = 0.00025). Therefore, CSF NFL increased in MSA patients relative to PD patients. Meta-regression showed that NFL was associated with α-synuclein in CSF of MSA patients relative to healthy controls. Due to the influence of heterogeneity sources, more prospective large sample studies are still needed to assess CSF NFL changes in MSA patients relative to PD patients.



Similar content being viewed by others
References
Ahmed Z, Asi YT, Lees AJ, Revesz T, Holton JL (2013) Identification and quantification of oligodendrocyte precursor cells in multiple system atrophy, progressive supranuclear palsy and Parkinson’s disease. Brain Pathol 23:263–273
Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T et al (2012) The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol 38:4–24
Stefanova N, Bucke P, Duerr S, Wenning GK (2009) Multiple system atrophy: an update. Lancet Neurol 8:1172–1178
Stefanova N, Kaufmann WA, Humpel C, Poewe W, Wenning GK (2012) Systemic proteasome inhibition triggers neurodegeneration in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. Acta Neuropathol 124:51–65
Kuzdas-Wood D, Stefanova N, Jellinger KA, Seppi K, Schlossmacher MG, Poewe W et al (2014) Towards translational therapies for multiple system atrophy. Prog Neurobiol 118:19–35
Shimohata T, Aizawa N, Nakayama H, Taniguchi H, Ohshima Y, Okumura H et al (2016) Mechanisms and prevention of sudden death in multiple system atrophy. Parkinsonism Relat Disord 30:1–6
Sako W, Murakami N, Izumi Y, Kaji R (2014) The difference in putamen volume between MSA and PD: evidence from a meta-analysis. Parkinsonism Relat Disord 20:873–877
Wang PS, Wu HM, Lin CP, Soong BW (2011) Use of diffusion tensor imaging to identify similarities and differences between cerebellar and Parkinsonism forms of multiple system atrophy. Neuroradiology 53:471–481
Laurens B, Constantinescu R, Freeman R, Gerhard A, Jellinger K, Jeromin A et al (2015) Fluid biomarkers in multiple system atrophy: a review of the MSA Biomarker Initiative. Neurobiol Dis 80:29–41
Bäckström DC, Eriksson Domellof M, Linder J, Olsson B, Ohrfelt A, Trupp M et al (2015) Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol 72:1175–1182
Sako W, Murakami N, Izumi Y, Kaji R (2015) Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson’s disease from atypical parkinsonism: evidence from a meta-analysis. J Neurol Sci 352:84–87
Sterne J, Moher D, and (editors)(2011) Chapter 10:addressing reporting biases.2011.In:Cochrane hand-book for systematic reviews of intervention version 510(updated March 2011)[Internet]. The Cochrane Collaboration Available from www.cochrane-handbool.org
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Holmberg B, Rosengren L, Karlsson JE, Johnels B (1998) Increased cerebrospinal fluid levels of neurofilament protein in progressive supranuclear palsy and multiple-system atrophy compared with Parkinson’s disease. Mov Disord 13:70–77
Holmberg B, Johnels B, Ingvarsson P, Eriksson B, Rosengren L (2001) CSF-neurofilament and levodopa tests combined with discriminant analysis may contribute to the differential diagnosis of Parkinsonian syndromes. Parkinsonism Relat Disord 8:23–31
Abdo WF, Bloem BR, Van Geel WJ, Esselink RA, Verbeek MM (2007) CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiol Aging 28:742–747
Constantinescu R, Rosengren L, Johnels B, Zetterberg H, Holmberg B (2010) Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson’s disease and atypical Parkinsonian disorders. Parkinsonism Relat Disord 16:142–145
Bech S, Hjermind LE, Salvesen L, Nielsen JE, Heegaard NH, Jorgensen HL et al (2012) Amyloid-related biomarkers and axonal damage proteins in parkinsonian syndromes. Parkinsonism Relat Disord 18:69–72
Hall S, Ohrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F et al (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 69:1445–1452
Herbert MK, Aerts MB, Beenes M, Norgren N, Esselink RA, Bloem BR et al (2015) CSF Neurofilament Light Chain but not FLT3 Ligand Discriminates Parkinsonian Disorders. Front Neurol 6:91. doi:10.3389/fneur.2015.00091
Magdalinou NK, Paterson RW, Schott JM, Fox NC, Mummery C, Blennow K et al (2015) A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry 86:1240–1247
Sacino AN, Brooks M, McKinney AB, Thomas MA, Shaw G, Golde TE et al (2014) Brain injection of alpha-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J Neurosci 34:12368–12378
Shea TB, Lee S (2011) Neurofilament phosphorylation regulates axonal transport by an indirect mechanism: a merging of opposing hypotheses. Cytoskeleton (Hoboken) 68:589–595
Teunissen CE, Khalil M (2012) Neurofilaments as biomarkers in multiple sclerosis. Mult Scler 18:552–556
Kuhle J, Gaiottino J, Leppert D, Petzold A, Bestwick JP, Malaspina A et al (2015) Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J Neurol Neurosurg Psychiatry 86:273–279
Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA et al (2016) Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron 91:56–66
Menke RA, Gray E, Lu CH, Kuhle J, Talbot K, Malaspina A et al (2015) CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol 2:748–755
Kang JH, Irwin DJ, Chen-Plotkin AS, Siderowf A, Caspell C, Coffey CS et al (2013) Association of cerebrospinal fluid beta-amyloid 1–42, T-tau, P-tau181, and alpha-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 70:1277–1287
Kang JH, Mollenhauer B, Coffey CS, Toledo JB, Weintraub D, Galasko DR et al (2016) CSF biomarkers associated with disease heterogeneity in early Parkinson’s disease: the Parkinson’s Progression Markers Initiative study. Acta Neuropathol 131:935–949
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Rights and permissions
About this article
Cite this article
Hu, X., Yang, Y. & Gong, D. Cerebrospinal fluid levels of neurofilament light chain in multiple system atrophy relative to Parkinson’s disease: a meta-analysis. Neurol Sci 38, 407–414 (2017). https://doi.org/10.1007/s10072-016-2783-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-016-2783-7