Abstract
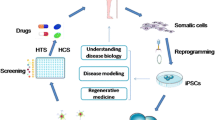
The study of stem-cell biology has been a flourishing research area because of its multi-differentiation potential. The emergence of induced pluripotent stem cells (iPSCs) open up the possibility of addressing obstructs, such as the limited cell source, inherent complexity of the human brain, and ethical constrains. Though still at its infancy phase, reprogramming of somatic cells has been demonstrating the ability to enhance in vitro study of neurodegenerative diseases and potential treatment. However, iPSCs would not thoroughly translate to the clinic before limitations are addressed. In this review, by summarizing the recent development of iPSC-based models, we will discuss the feasibility of iPSC technology on relevant diseases depth and illustrate how this new tool applies to drug screening and celluar therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases occur as a result of neurodegenerative processes, including chronic and progressive loss of structure or function of neurons. Compared with other organs, recent progress in the molecular basis of neurodegenerative disorders has evolved slowly. This mainly due to brain tissue samples were rarely available for obtaining and often demonstrated the final phase of the diseases. These limitations impede our progress for better understanding of disease onset and mechanisms [1]. Although genetically engineered animal models have achieved promising advances, these systems are still inadequate and do not faithfully mirror the disease mechanism due to species differences and genetic backgrounds. Possessing the developmental potential to form trophoblast and differentiate into three embryonic germ layers, human embryonic stem cells (hESCs) could be a new paradigm [2]. However, the success rate of hESC establishment is extremely low, combining with lacking of oocyte donors and ethical issues further restricted the extensive application. The recent method of obtaining induced pluripotent stem cells (iPSCs) from somatic cells offer unprecedented and exciting opportunities to solve multiple drawbacks of various models mentioned above. The aim of this review is to assess the recent literatures and key findings on modeling neurodegenerative disease using iPSCs. The potential of iPSCs as an ideal platform for drug screening and cell therapy are presented. Besides, limitations and challenges in iPSC modeling will also be discussed.
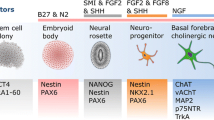
The developmental process of iPSC technology
Yamanaka first illustrated how cell fates rewound to a pre-embryonic state using retroviruses and lentiviruses by the ectopic co-expression of transcription factors [3, 4]. That discovery won him the Nobel Prize for Medicine in 2012. These iPS cells subsequently converted to specific cells of interest, such as neurons and glia that are relevant for different neurodegenerative diseases. However, it is also time-consuming, expensive. Moreover, this new approach possesses the possibility of oncogene reactivation when the cocktail of reprogramming factors contained proto oncogene c-MYC and the risk of insertional mutations. To reduce the potential adverse effects and to emphasize the need to safety, hiPSCs have been established by excluding c-MYC of four conventional reprogramming factors [5, 6]. Several groups took advantages of adenoviral and plasmid for non-integrating which avoid tumor formation, however, these manners are very labor intensive and the efficiency in general is extremely low [7, 8]. Sendai virus, protein, modified mRNA, and Micro-RNA have been used to generate iPSCs with no genomic integration [9–13]. The disadvantages of each vector type are still apparent. For example, the problems of purging cells of replicating virus and sequence-sensitive RNA replicase when applying Sendai virus can not be ignored [13]. Multiple rounds of transfection using modified mRNA vector were needed to achieve controllable and high-efficiency goal [12]. Recently, researchers have obtained mouse iPSCs from somatic cells using a combination of small-molecule compounds because they are cost-effective, easily synthesized, and nonimmunogenic [14]. Important progresses of iPSC-based scientific exploration have already been made on neurological diseases, haematological diseases, cardiac diseases, liver related diseases, etc. Here, we focus on current-established iPSCs models on neurodegenerative disorders (summarized in Table 1).
Parkinson’s disease
Parkinson’s disease (PD) is the most common form of a group of progressive neurodegenerative movement disorders, and the prevalence is about 100–300 per 100,000 [15]. Initial PD-iPS cells were generated to reveal aspects of the abnormalities of PD, but no relevant phenotype was evident in the study [16]. Later, iPSC lines from a patient harboring a mutation in LRRK2 gene were found partially consistent with early PD phenotype of enhanced susceptibility to oxidative stress (OS) [17]. In LRRK2 G2019S iPSC-derived dopaminergic (DA) neurons, morphological alterations were observed when compared to control lines, with neurons showing decreased neurite length and reduced numbers of neuritis. This study also observed accumulation of autophagosomes and reduction of autophagic flux [18]. Recently, the phenotypes in two patient-derived iPSC lines with LRRK2 G2019S mutation were rescued by specifically correcting the corresponding mutant allele. Moreover, certain experiment highlighted an elevated level of α-synuclein and MAPT expression in counterpart lines while not in the LRRK2 genetically corrected lines [19]. Mutations in either PINK1 or PARK2 cause recessive forms of inherited PD characterized by impaired mitophagy because PINK1 and Parkin proteins function together. A study, including three patients with missense (c.509T>G; p.V170G) in mitochondrial protein PINK1, reported that the PINK1 mutant phenotype could be reversed after overexpression of wild-type PINK1 [20]. In studies examining iPSC cells from patients with PARK2 mutations, iPSC-derived neurons demonstrated functional and morphological abnormalities of mitochondria along with increased oxidative stress, rather than iPSCs or fibroblasts [21]. Similar to Imaizumi and colleagues, Jiang et al. [22] demonstrated altered features in mutant DA neurons, including enhanced sensibility to OS and monoamine oxidases activity, increased spontaneous DA release, and decreased DA uptake. These phenotypes were reversed through lentiviral expression of Parkin. Human iPSC-derived lines with mutations in SNCA expressed double the amount of α-synuclein when differentiated into DA neurons, while not seen in the original fibroblasts [23]. By use of zinc finger nuclease-mediated genome editing technology, researchers corrected the underlying point mutations (A53T) in SNCA-PD-iPSC. However, the phenotypes of iPSC-derived neurons in this work were not assessed [24]. A more recent study applied iPSCs possessing GBA1 mutations to the modeling of PD. Schondorf et al. [25] observed increased levels of α-synuclein and glucosylceramide as well as lysosomal and autophagic defects in neurons from GBA1 PD-iPSC. Importantly, these pathological phenotypes could be rescued by correction of the mutations.
Alzheimer’s disease
Alzheimer’s disease (AD) is caused by progressive degeneration and loss of neurons and synapses throughout the brain [26]. Most of early-onset familial AD (FAD) can be attributed to mutations in one of three genes: APP, PS1 and PS2, while many genetic and environmental factors may co-contribute to determining the sporadic AD (SAD) [27]. The investigation of AD was extremely limited until FAD-derived iPSCs with PS1 (A246E) and PS2 (N141I) mutations were established. In FAD-iPSC-derived differentiated neurons, the secretion of amyloid-beta (Aβ) was significantly increased and sharply responds to γ-secretase modulators and inhibitors [28]. Furthermore, iPSC-derived neuronal cells showed AD-like biochemical features, such as an increase in Aβ ratio. These neurons have reduced to secrete Aβ when treated with β-secretase inhibitor, γ-secretase inhibitor, and a nonsteroidal antiinflammatory drug. During the differentiation stages, however, there existed different susceptibilities to these drugs [29]. Other iPSCs model was established from FAD patients with a duplication of APP gene and two cases of SAD. Higher level of phospho-tau and glycogen synthase kinase-3 in lines with elevated Aβ represented novel observations in neurons from one of the SAD cases. Interestingly, these alterations could be alleviated by β-secretase, not γ-secretase, inhibitor treatment [30]. Docosahexaenoic acid (DHA) may actually be effective for some patients for the reason that the stress responses in the AD neural cells were alleviated when treated with DHA, making possible the iPSC technology for validation and identification of promising drugs [31].
Huntington’s disease
Huntington’s disease (HD) is a progressive neurodegenerative genetic disease associated with a wide variety of motor impairment and psychiatric symptoms caused by excessive CAG trinucleotide expansions of Huntingtin gene (HTT) [32]. The first established human HD-iPSC model from HD patients carrying 72 CAG repeats was reported in 2008 [33]. Striatal neurons and HD neural stem cells produced from iPSCs exhibited elevated caspase activity after the withdrawal of growth factors which indicate apoptosis, but no overt cell death phenotype was reported [34]. Experimental analysis of proteins revealed amounts of distinct alternations in protein expression in the HD iPSCs [35]. Jeon et al. converted HD-iPSC into GABAergic striatal neurons and relavent behavior recovered in a grafted rat after transplantation of HD-iPSC derived neural precursors. Though showing no overt HD phenotype, the disease-specific iPSCs sharply responded to proteasome inhibition and expressed several markers of HD pathology after transplantation at later time points [36, 37]. Additional HD-phenotypes, including altered mitochondrial bioenergetics and susceptibility to cell death were reported in a subsequent study. These alterations accompanied with pathogenic HD signaling pathways could be reversed by the substitution of a normal repeat for the expanded CAG repeat using homologous recombination [38]. A more complete study by the HD Consortium demonstrated hundreds of distinct differences between HD and wide-type (WT) iPSCs, showing clear signs of HD-related pathology in several lineages. The lines with the longer CAG repeat expansions had more severe pathological phenotypes and ultimately lead to neuronal death [39]. These findings revealed that HD-iPSCs could be a well-characterized and unique resource to elucidate disease mechanisms.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is the most common form of progressive motor neuron disorder with varied etiology characterized by rapidly progressive weakness and muscle atrophy. This disease strikes people in their 40s, approximately 20 % of familial ALS cases were linked to dominant mutations in the SOD1 gene [40]. Other different genes, including VAPB, TDP-43 and FUS have also been implicated in ALS [41, 42]. ALS-iPSCs were developed using skin fibroblasts from two octogenarian sisters with SOD1 mutations and then converted into spinal motor neurons, but no assay of ALS relevant phenotype or compared with neurons from controls were performed in this study [43]. Another iPSC-derived motor neurons (MNs) possessing SOD1 mutations developed neurofilament (NF) inclusions. Importantly, conditional expression of NF-L in these MNs corrected the NF subunit proportion, mitigating NF aggregation and neurite degeneration [44]. The mutant astrocytes from iPSCs harboring a mutation in the TDP-43 gene exhibited subcellular mislocalization of TDP-43, increased levels of TDP-43, and reduced cell survival. Further co-culture assays showed these astrocytes did not adversely affect survival of cocultured neurons [45]. Another TDP-43 iPSC-derived MNs recapitulated the disease phenotypes, including decreased survival, increased cellular vulnerability, and elevated levels of soluble and detergent-resistant TDP-43 protein [46]. Researchers also generated iPSCs from patients which carry the VAPB mutation as well as from their healthy siblings. The finding suggested that reduced levels of VAPB protein in MNs could be involved in the pathogenesis of ALS8 [47]. Test for chemical compounds on differentiated MNs showed that the abnormal ALS MNs phenotype could be rescued by anacardic acid, suggesting that special MNs may be a new approach for elucidating ALS disease mechanisms and for screening candidate drugs [48].
Spinal muscular atrophy
Spinal muscular atrophy (SMA) is an autosomal recessive genetic disease characterized by the loss of the survival motor neuron (SMN) [49]. There are two SMN genes in humans, SMN1 and SMN2, and the loss of the SMN1 gene primarily caused SMA [50]. To model SMA, scientists generated iPSCs from a child carrying SMN1 mutation. These cells manifested clear signs of motor neurons and differentiated neurons showed the reduction in cells size and number compared to controls at later stages, indicating they underwent substantial degeneration. Excitingly, the administration of valproic acid and tobramycin could reverse the endogenous SMN protein level in differentiated neurons and astrocytes [51]. Another established neuronal cultures rescued the phenotype of delayed neurite outgrowth and restored normal motoneuron differentiation by overexpressing SMN, accelerating the exploration of the underlying mechanisms of SMA pathogenesis [52]. MNs from two type I SMA subject-derived iPSCs showed elevated activation of caspase-8 and-3 and increased Fas ligand-mediated apoptosis. Moreover, distinct inhibitors of apoptotic pathways may reduce motor neuron cell death [53]. Another study has focused on genetic correction for autologous cell therapy. Uncorrected SMA-iPSCs derived motor neurons manifested disease-specific features. Delightfully, these phenotypes were ameliorated in genetically corrected controls, suggesting that genetically corrected special motor neurons could be a source for future therapeutic strategy [54]. Novel observations of reduced androgen receptor levels, reduced HDAC6, and repeat instability provided evidence that SMA-iPSC derived MNs could be new avenues for further investigation of the disease mechanism and development of effective therapy [55].
Inherited ataxias
Inherited ataxias may show autosomal dominant, autosomal recessive modes of inheritance. Friedreich ataxia (FRDA) is an autosomal recessive ataxia, accounting for one-half of all hereditary ataxias. Spinocerebellar ataxias (SCAs) are genetically defined autosomal dominantly inherited disorders characterized by progressive lack of motor coordination.
FRDA is caused by large guanine-adenine-adenine (GAA) expansions in FXN gene on chromosome 9q13, leading to a transcriptional defect of FXN mRNA and frataxin [56, 57]. GAA·TTC triplet repeats in FXN intron 1 in FRDA iPSCs not only expanded at a higher rates but also exhibited repeat instability [58, 59]. The mismatch repair enzymes MSH2 were much highly expressed in iPSCs than fibroblasts and neuronal stem cells. Moreover, a specific pyrrole-imidazole polyamide which displaced MSH2 in FRDA iPSCs could partially impede repeat expansion [59]. Studies above demonstrated that GAA repeats instability might start early during ontogenesis and the highly active mismatch repair system is related to the GAA·TTC triplet repeats. Another iPSCs presented an instable repeats of GAA expansion, but was failed to find biochemical phenotypes [60]. Recently, a new development-based differentiation protocol was applied to differentiate FRDA and control iPSCs into peripheral sensory neurons and neural crest progenitors. Increased expression of frataxin during sensory specification for control cells was identified compared to FRDA peripheral sensory neurons. Whereas, a pronounced deficiency of frataxin was observed in FRDA iPSCs and neural crest cells, rather than controls [61].
SCA3, also known as Machado-Joseph disease, is the most common subtype of SCAs in China, which is caused by an abnormal CAG trinucleotide repeat expansion in ATXN3 gene [62]. The formation of sodium dodecyl sulfate insoluble aggregates was observed in l-glutamate-induced excitation of iPSC-derived neurons and calpain inhibition could abolish these pathological changes. However, inclusion bodies and increased cell death were not observed, suggesting the excitation-induced protein aggregation is an early event [63].
Familial dysautonomia
Familial dysautonomia (FD) is a debilitating neurodegenerative disease and the most common mutation is in a splice site of the IKBKAP gene involved in transcriptional elongation, resulting in reduced levels of IKAP protein [64]. Researchers differentiated patient-specific FD-iPSCs into peripheral neurons directly. Tissue-specific mis-splicing of IKBKAP in vitro and low level of normal IKBKAP transcript expressed in patient neural crest precursors were observed. The potency treatment of rescuing the mutant splice combines with improving neuronal differentiation and migration were also concluded [65, 66]. Later, 6,912 compounds were screened, while 8 hits were characterized that rescued expression of IKBKAP. SKF-86466, one of 8 hits, not only induced IKBKAP transcription but also rescued the expression of IKAP protein and the disease-specific loss of autonomic neuron sign [67]. Taken together, small molecule discovery in these newly disease models can gain novel insights into human disease pathogenesis and promising treatment.
Potential and challenges
The promising iPSC technology has the potential to model and treat neurodegenerative diseases. Patient-derived iPSCs can be rewound to affected neuronal subtypes via in vitro differentiation or repaired iPS cells using gene targeting to repair disease-causing mutation. One notable potential is to apply these affected neuronal subtypes to develop promising drugs that are most suitable for the patient associated with their efficacy and toxicity profile. The generation of iPSCs permits the production of large scale of central nervous system cell with specific genotype, providing an unlimited amount of experimental materials. The other potential is to develop autologous-repaired iPS cells for cell therapy. When transplanting desired cells into patients, neural function could be restored and long-term immuno-suppression may not be necessary. Moreover, transplantation of glial cells, such as astrocytes that would restore the motor neurons potentially, can also used for neuroprotection.
However, it is not until this technology ready for clinical applications that its limitations were overcame. Firstly, initial method that introduction of factors by retrovirus or lentivirus might provide the possibility of oncogene reactivation [3, 4]. Apart from safety, the genetic and epigenetic status of clones might have varied after reprogramming. Although the non-integrating approaches significantly reduced the risk of tumorigenesis and epigenetic variation, researchers need to be cautious and select the stable lineages for differentiation studies. Epigenetic modifications could be significant in disease manifestation and the erasure of epigenetic marks during reprogramming is important [7–13]. Secondly, how to generate disease-relevant phenotype in vitro is also a challenge. The appearance of disease phenotype is the most important feature for disease modeling. Current iPSC-models were generated from patients containing mutations in known gene. However, many patients, such as PD and ALS, have an unidentified genetic component that is coupled with environmental factors. Importantly, the majority of neurodegenerative disorders are age-dependent and age is considered to be a risk factor that contributes to the disease development. As current culture system involved no disease-triggering factors on disease, the iPSCs might no longer reflect relevant pathogenesis. Moreover, long-term culture was poorly controlled and could be compromised by excessive cell death. Thirdly, protocols for differentiating iPSCs towards different subtypes of neurons cannot be ignored. Up till now, greater progress has been made in generating matured populations of distinct neurons and glia for the purpose of screening drugs and replacement therapy. However, these cells may not truly reflect the cellular responses to compounds that the body would have at a physiological level. Lastly, researchers still face the problems of low efficiency conversion and laborious to conduct. The average conversion efficiency of each methods mentioned above is less than 1 %, which further restrict the extensively use of the technology [1]. Efficiency needs to be improved if these protocols apply for high-throughout drug screening.
Conclusions
Taken together, iPSC technology for modeling of neurodegenerative diseases has both benefits and limitations. Patient-derived cells open new avenues to investigate the neurodegenerative diseases development and remarkably easily could usher in new therapies and cloning techniques. Many hurdles must be overcome before new clinical therapies based on these cells. This will help ensure safety and eventually beneficial to human beings.
References
Robinton DA, Daley GQ (2012) The promise of induced pluripotent stem cells in research and therapy. Nature 481:295–305
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Huangfu D, Osafune K, Maehr R et al (2008) Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 26:1269–1275
Nakagawa M, Koyanagi M, Tanabe K et al (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26:101–106
Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S (2008) Generation of mouse induced pluripotent stem cells without viral vectors. Science 322:949–953
Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K (2008) Induced pluripotent stem cells generated without viral integration. Science 322:945–949
Zhou H, Wu S, Joo JY et al (2009) Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4:381–384
Heng BC, Heinimann K, Miny P et al (2013) mRNA transfection-based, feeder-free, induced pluripotent stem cells derived from adipose tissue of a 50-year-old patient. Metab Eng 18:9–24
Wang T, Shi SB, Sha HY (2013) Micro RNAs in regulation of pluripotency and somatic cell reprogramming: small molecule with big impact. RNA Biol 10:1255–1261
Warren L, Manos PD, Ahfeldt T et al (2010) Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7:618–630
Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M (2009) Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci 85:348–362
Hou P, Li Y, Zhang X et al (2013) Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 341:651–654
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373:2055–2066
Soldner F, Hockemeyer D, Beard C et al (2009) Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136:964–977
Nguyen HN, Byers B, Cord B et al (2011) LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8:267–280
Sanchez-Danes A, Richaud-Patin Y, Carballo-Carbajal I et al (2012) Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med 4:380–395
Reinhardt P, Schmid B, Burbulla LF et al (2013) Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell 12:354–367
Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D (2011) Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci 31:5970–5976
Imaizumi Y, Okada Y, Akamatsu W et al (2012) Mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain 5:35
Jiang H, Ren Y, Yuen EY et al (2012) Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced pluripotent stem cells. Nat Commun 3:668
Devine MJ, Ryten M, Vodicka P et al (2011) Parkinson’s disease induced pluripotent stem cells with triplication of the alpha-synuclein locus. Nat Commun 2:440
Soldner F, Laganiere J, Cheng AW et al (2011) Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146:318–331
Schondorf DC, Aureli M, McAllister FE et al (2014) iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun 5:4028
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Alzheimer’s disease. Lancet 377:1019–1031
Piaceri I, Nacmias B, Sorbi S (2013) Genetics of familial and sporadic Alzheimer’s disease. Front Biosci (Elite Ed) 5:167–177
Yagi T, Ito D, Okada Y et al (2011) Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet 20:4530–4539
Yahata N, Asai M, Kitaoka S et al (2011) Anti-Abeta drug screening platform using human iPS cell-derived neurons for the treatment of Alzheimer’s disease. PLoS One 6:e25788
Israel MA, Yuan SH, Bardy C et al (2012) Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482:216–220
Kondo T, Asai M, Tsukita K et al (2013) Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Abeta and differential drug responsiveness. Cell Stem Cell 12:487–496
Ha AD, Fung VS (2012) Huntington’s disease. Curr Opin Neurol 25:491–498
Park IH, Arora N, Huo H et al (2008) Disease-specific induced pluripotent stem cells. Cell 134:877–886
Zhang N, An MC, Montoro D, Ellerby LM (2010) Characterization of human Huntington’s disease cell model from induced pluripotent stem cells. PLoS Curr 2:Rrn1193
Chae JI, Kim DW, Lee N et al (2012) Quantitative proteomic analysis of induced pluripotent stem cells derived from a human Huntington’s disease patient. Biochem J 446:359–371
Jeon I, Lee N, Li JY et al (2012) Neuronal properties, in vivo effects, and pathology of a Huntington’s disease patient-derived induced pluripotent stem cells. Stem Cells 30:2054–2062
Jeon I, Choi C, Lee N et al (2014) In vivo roles of a patient-derived induced pluripotent stem cell line (HD72-iPSC) in the YAC128 model of Huntington’s disease. Int J Stem Cells 7:43–47
An MC, Zhang N, Scott G et al (2012) Genetic correction of Huntington’s disease phenotypes in induced pluripotent stem cells. Cell Stem Cell 11:253–263
HD iPSC Consortium (2012) Induced pluripotent stem cells from patients with Huntington’s disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell 11:264–278
Turner MR, Hardiman O, Benatar M et al (2013) Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol 12:310–322
Aliaga L, Lai C, Yu J et al (2013) Amyotrophic lateral sclerosis-related VAPB P56S mutation differentially affects the function and survival of corticospinal and spinal motor neurons. Hum Mol Genet 22(21):4293–4305
Al-Chalabi A, Jones A, Troakes C, King A, Al-Sarraj S, van den Berg LH (2012) The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol 124:339–352
Dimos JT, Rodolfa KT, Niakan KK et al (2008) Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321:1218–1221
Chen H, Qian K, Du Z et al (2014) Modeling ALS with iPSCs reveals that mutant SOD1 misregulates neurofilament balance in motor neurons. Cell Stem Cell 14:796–809
Serio A, Bilican B, Barmada SJ et al (2013) Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc Natl Acad Sci USA 110:4697–4702
Bilican B, Serio A, Barmada SJ et al (2012) Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability. Proc Natl Acad Sci USA 109:5803–5808
Mitne-Neto M, Machado-Costa M, Marchetto MC et al (2011) Downregulation of VAPB expression in motor neurons derived from induced pluripotent stem cells of ALS8 patients. Hum Mol Genet 20:3642–3652
Egawa N, Kitaoka S, Tsukita K et al (2012) Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Trans Med 4:145ra104
Hamilton G, Gillingwater TH (2013) Spinal muscular atrophy: going beyond the motor neuron. Trends Mol Med 19:40–50
Nurputra DK, Lai PS, Harahap NI et al (2013) Spinal muscular atrophy: from gene discovery to clinical trials. Ann Hum Genet 77(5):435–463
Ebert AD, Yu J, Rose FF Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN (2009) Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457:277–280
Chang T, Zheng W, Tsark W, Bates S, Huang H, Lin RJ, Yee JK (2011) Brief report: phenotypic rescue of induced pluripotent stem cell-derived motoneurons of a spinal muscular atrophy patient. Stem Cells 29:2090–2093
Sareen D, Ebert AD, Heins BM, McGivern JV, Ornelas L, Svendsen CN (2012) Inhibition of apoptosis blocks human motor neuron cell death in a stem cell model of spinal muscular atrophy. PLoS One 7:e39113
Corti S, Nizzardo M, Simone C et al (2012) Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Sci Trans Med 4:165ra162
Grunseich C, Zukosky K, Kats IR et al (2014) Stem cell-derived motor neurons from spinal and bulbar muscular atrophy patients. Neurobiol Dis 70:12–20
Koeppen AH (2011) Friedreich’s ataxia: pathology, pathogenesis, and molecular genetics. J Neurol Sci 303:1–12
Lufino MM, Silva AM, Nemeth AH, Alegre-Abarrategui J, Russell AJ, Wade-Martins R (2013) A GAA repeat expansion reporter model of Friedreich’s ataxia recapitulates the genomic context and allows rapid screening of therapeutic compounds. Hum Mol Genet 22(25):5173–5187
Ku S, Soragni E, Campau E et al (2010) Friedreich’s ataxia induced pluripotent stem cells model intergenerational GAATTC triplet repeat instability. Cell Stem Cell 7:631–637
Du J, Campau E, Soragni E, Ku S, Puckett JW, Dervan PB, Gottesfeld JM (2012) Role of mismatch repair enzymes in GAA.TTC triplet-repeat expansion in Friedreich ataxia induced pluripotent stem cells. J Biol Chem 287:29861–29872
Hick A, Wattenhofer-Donze M, Chintawar S et al (2013) Neurons and cardiomyocytes derived from induced pluripotent stem cells as a model for mitochondrial defects in Friedreich’s ataxia. Dis Model Mech 6:608–621
Eigentler A, Boesch S, Schneider R, Dechant G, Nat R (2013) Induced pluripotent stem cells from friedreich Ataxia patients fail to up-regulate frataxin during in vitro differentiation to peripheral sensory neurons. Stem Cells Dev 22(24):3271–3282
Costa Mdo C, Paulson HL (2012) Toward understanding Machado-Joseph disease. Prog Neurobiol 97:239–257
Koch P, Breuer P, Peitz M et al (2011) Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature 480:543–546
Slaugenhaupt SA, Blumenfeld A, Gill SP et al (2001) Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet 68:598–605
Lee G, Papapetrou EP, Kim H et al (2009) Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461:402–406
Lee G, Studer L (2011) Modelling familial dysautonomia in human induced pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci 366:2286–2296
Lee G, Ramirez CN, Kim H et al (2012) Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat Biotechnol 30:1244–1248
Acknowledgments
This work was supported by research grants from the Hunan Provincial Natural Science Foundation of China (13JJ2014) and National Natural Science Foundation of China (81071001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Xie, Y.Z., Zhang, R.X. Neurodegenerative diseases in a dish: the promise of iPSC technology in disease modeling and therapeutic discovery. Neurol Sci 36, 21–27 (2015). https://doi.org/10.1007/s10072-014-1989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1989-9