Abstract
Diabetes mellitus (DM) is associated with an increased risk of mild cognitive impairment, dementia and stroke. The association between DM and dementia appears to be stronger for vascular cognitive impairment than for Alzheimer’s disease, suggesting cerebrovascular disease may be an important factor in cognitive impairment in DM. Although the exact mechanisms by which DM affects the brain remain unclear, changes to brain vasculature, disturbances of cerebral insulin signaling, insulin resistance, glucose toxicity, oxidative stress, accumulation of advanced glycation end products, hypoglycemic episodes, and alterations in amyloid metabolism may all be involved. Cognitive impairment and dementia associated with DM may also be mediated via vascular risk factors, in particular brain ischemia, the occurrence of which can have an additive or synergistic effect with concomitant neurodegenerative processes. To date, no drug has been approved for the treatment of vascular dementia and there are no specific pharmacological treatments for preventing or reducing cognitive decline in patients with DM. Most focus has been on tighter management of vascular risk factors, although evidence of reduced cognitive decline through reducing blood pressure, lipid-lowering or tighter glycemic control is inconclusive. Tailored, multimodal therapies may be required to reduce the risk of cognitive dysfunction and decline in patients with DM. The use of pleiotropic drugs with multimodal mechanisms of action (e.g., cerebrolysin, Actovegin) may have a role in the treatment of cognitive dysfunction and their use may warrant further investigation in diabetic populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is associated with an increased risk of mild cognitive impairment, dementia, and stroke [1]. DM is likely to become an increasingly important contributory factor in dementia, especially given an estimated global population of 552 million affected individuals by 2030 [2].
Vascular brain pathology underlying cognitive decline is heterogeneous and can involve a variety of processes leading to acute or chronic ischemia or a combination of both. Although Alzheimer’s disease (AD) is generally considered the most frequent dementia diagnosis, vascular cognitive decline may be more common than previously believed. However, it can be difficult to distinguish between the two and most patients, particularly in old age, will have mixed dementia [3]. Several cohort studies have shown mixed pathology on autopsy in the majority of dementia patients, including AD changes (e.g., amyloid-beta plaques) and cerebrovascular lesions (infarcts, lacunas, microbleeds and white matter lesions) [4].
Diabetes and cognitive function
Several studies have shown that DM is a risk factor for cognitive impairment and dementia [5, 6]. These mostly focus on type 2 rather than type 1 DM, which accounts for the majority of DM patients (at least 90 %). Cross-sectional studies have generally shown worse cognitive performance in patients with DM compared with matched controls [7]. Longitudinal studies have also reported accelerated cognitive decline in patients with DM [8, 9]. Two studies have recently reported that higher glucose levels may be a risk factor for cognitive impairment or dementia even among persons without DM [10, 11]. Modest cognitive decrements are already present in patients with early-stage type 2 DM [12] and metabolic syndrome has been reported to affect cognition and raise the risk of dementia [13]. However, evidence supporting a causal association between DM and cognitive impairment is mixed.
The association between DM and dementia appears to be stronger for vascular cognitive impairment than for AD. A recent meta-analysis reported that DM was associated with an increased relative risk of 1.2 for mild cognitive impairment, 1.5 for AD and 2.5 for vascular dementia [14]. Elderly patients with DM have also been reported to have a reduced amyloid-beta load and more cerebral infarcts versus non-diabetics [15].
DM may be associated with modest cognitive decrements in non-demented individuals that progress only slowly over time, causing subtle changes to self-esteem, mood, and wellbeing. DM may also be associated with an increased risk of more severe cognitive deficits and dementia in certain patients. These two processes may reflect a continuum with modest impairment at an earlier stage; however, a distinction between these two types has been noted with regard to age groups and trajectories of development, and it has been suggested that these may reflect separate processes [16]. If so, these two processes may be associated with different risk factors and potentially require different treatments.
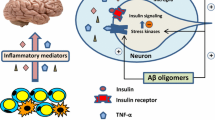
A wide range of metabolic and vascular disturbances have been implicated in the pathophysiology of cognitive impairment (Fig. 1) [17]. The exact mechanisms by which DM affects the brain remain unclear but probably involve both cerebrovascular and neurodegenerative changes. Changes to brain vasculature, disturbances of cerebral insulin signaling, insulin resistance, glucose toxicity, oxidative stress, accumulation of advanced glycation end products, hypoglycemic episodes, and alterations in amyloid metabolism may all be involved. Neuroimaging studies have shown structural changes to the brain in patients with DM, with magnetic resonance imaging (MRI) studies showing an association between DM and silent and asymptomatic brain infarcts [18]. DM has also been reported to be a risk factor for white matter lesion (WML) progression [19], although this relationship is less consistent.
Potential causes of cognitive impairment in type 2 diabetes. Adapted from [17]
Diabetes and acute ischemic stroke
Cognitive impairment and dementia associated with DM may be mediated via ischemic stroke, which can have an additive or synergistic effect with concomitant neurodegenerative lesions. Patients with DM are well recognized as being at increased risk of stroke. In a meta-analysis involving almost 700,000 patients, DM is more than doubled the risk of ischemic stroke after adjusting for body mass index, blood pressure, lipids, and other risk factors (hazard ratio 2.27; 95 % CI 1.95–2.65) [20]. DM is also associated with worse outcomes in stroke patients, in particular increased mortality [21].
There is a high rate of previously undiagnosed DM in acute stroke patients. In one cohort of 238 acute stroke patients, 36 % had DM, including 16 % with newly diagnosed DM. An additional 24 % had impaired glucose tolerance or abnormally high fasting glucose [22]. The proportion of acute stroke patients with previously unknown DM rather than transient stress hyperglycemia may be higher than is often thought, especially since criteria for defining DM in stroke trials are typically a history of DM or intake of anti-diabetes medications [23].
Hyperglycemia during the acute phase of stroke is associated with worse short-term outcomes. In a systematic review, relative risk of in-hospital or 30-day mortality after an ischemic stroke was 3.3 in hyperglycemic patients without known DM and 2.0 in those with known DM when compared to patients with normoglycemia [24]. In an analysis of 268 patients with a non-lacunar stroke, admission hyperglycemia was negatively correlated with the degree of neurological improvement at 24 h in reperfused but not non-reperfused recombinant tissue plasminogen activator (rt-PA)-treated patients [25], suggesting the deleterious effect of hyperglycemia on infarct growth may be related to whether or not reperfusion occurs. However, in another trial, higher admission glucose levels were associated with decreased likelihood of neurological improvements and increased risk of symptomatic intracerebral hemorrhage, regardless of rt-PA treatment [26].
Several mechanisms have been suggested by which hyperglycemia may have a deleterious effect in ischemic stroke, including impaired recanalization and increased reperfusion injury (Fig. 2) [27]. The association between hyperglycemia and poor outcome after stroke is mainly related to large-vessel infarction and is less evident in lacunar stroke [28]. This may be because hyperglycemia reduces salvage of the penumbra, the ischemic area that can potentially recover if adequate reperfusion is administered within hours of stroke onset. A penumbra is not usually present in lacunar stroke.
Potential effects of hyperglycaemia over time on pathological development of cerebral infarction (reproduced with permission from [27])
The deleterious effect of hyperglycemia on stroke outcomes raises the question of whether glucose-lowering treatment during the acute phase of stroke may be beneficial. In other critically ill (non-stroke) patients with hyperglycemia, initial studies suggested that intensive insulin therapy could be beneficial [29]. However, later studies failed to confirm these findings. In the NICE-SUGAR study, intensive glucose control increased mortality among adults in intensive care, with a blood glucose target of 180 mg/dL or less resulting in lower mortality than a target of 81–108 mg/dL [30]. Moreover, more intensive glucose-lowering treatment may be associated with an increased risk of severe hypoglycemia [31].
There is no evidence to date that glucose-lowering improves clinical outcomes in acute stroke. In the GIST-UK study, patients presenting within 24 h of stroke onset randomized to variable-dose glucose–potassium–insulin had significantly reduced plasma glucose concentration and systolic blood pressure but no significant reduction in mortality at 90 days compared with the control group [32]. However, it should be noted that the trial was underpowered, over 20 % of patients had lacunar stroke and glucose levels during the 24-h treatment period were only 0.57 mmol/L lower in the intensive treatment group. In a pilot study in which patients were randomized to intensive insulin (target glucose <7.2 mmol/L) or standard insulin treatment (target glucose <11.1 mmol/L), clinical outcomes were considered somewhat better with intensive therapy, although differences between groups were not significant [33]. A definitive phase III trial (SHINE) is currently underway to compare standard of care glucose control to an intensive level of control in hyperglycemic acute ischemic stroke patients [34].
Treatment options for preventing or reducing cognitive decline
To date, there are no pharmacological treatments for preventing or reducing cognitive decline in patients with DM. Most focus has been on tighter management of vascular risk factors to help ameliorate cognitive decline. Anti-hypertensive therapy has been reported to reduce dementia risk in the general population, although results from randomized, controlled studies are inconclusive [35]. In the PROGRESS trial of 6,105 individuals with prior stroke or transient ischemic attack, cognitive decline was significantly less with perindopril (with or without indapamide) compared with placebo, and there was a non-significant decrease in incident dementia [36]. Similarly, the HYVET-Cog study on indapamide with or without perindopril showed a non-significant effect on incident dementia of 0.86 (95 % CI 0.67–1.09) [37]. Other studies reported no benefit of anti-hypertensive treatment on cognitive performance or dementia incidence [38, 39]. The efficacy of lipid-lowering therapy in reducing cognitive decline is also not proven, with no effect seen with simvastatin [40] or pravastatin [41]. Several methodological issues may hinder the ability to demonstrate reduced cognitive decline through vascular risk factor reduction, including patients being relatively young with low incidence of cognitive dysfunction, insufficient follow-up, high dropout rates due to cognitive impairment, and additional risk factor intervention in the placebo/control group [35].
In patients with DM, the effect of tighter glycemic control on cognitive function is inconsistent. Some studies have reported a benefit of improved control on cognitive decline [42, 43] while others have shown no difference [44]. In the ADDITION study, cognitive decline in patients with screening-detected type 2 DM did not differ between intensive multifactorial treatment and routine care after 6 years [45]. Moreover, cognitive decline in both groups was within the range observed in a control group of non-diabetic study participants. In the largest trial reported to date, the ACCORD-MIND study, cognitive function at 40 months was not improved with intensive (HbA1c <6.0 %) compared with standard glycemic control (HbA1c 7.0–7.9 %) [46]. However, decline in total brain volume was significantly reduced in the intensive therapy group (Fig. 3). Structural changes in the brain may occur before cognitive differences between groups are apparent and longer-term follow-up may be needed to detect a benefit of more intensive control. It has also been suggested that mean cognitive performance was relatively stable over time in both groups, leaving little room for a treatment effect [6]. However, given the increased mortality observed in patients with intensive treatment, tighter glycemic control is not recommended to reduce the adverse effects of DM on the brain. Moreover, a history of severe hypoglycemic episodes has also been associated with increased dementia, suggesting any benefits of tighter control may need to be balanced against a higher hypoglycemia risk [47].
Outcomes in the ACCORD-MIND study [46]. a Digit symbol substitution test (DSST). b Total brain volume
The effects of oral anti-diabetic drugs in the prevention and treatment of vascular cognitive impairment are still to be clarified. Some studies initially suggested that thiazolidinediones may have a beneficial impact on cognitive function in patients with AD [48]. However, larger, more robust trials of rosiglitazone failed to show any benefit [49]. Data from the ACCORD-MIND study suggested that treatment with rosiglitazone was associated with increased cognitive decline after 40 months [50], although these results may have been confounded by unexplained differences between patients. No relationship between insulin use and cognitive function was observed. Metformin has been associated with an increase in amyloid peptides in neuronal cultures, which raises the possibility that metformin may accelerate clinical manifestations of AD in patients with type 2 DM [51]. In a recent retrospective study, metformin use was associated with worse cognitive performance among patients with DM [52]. However, animal studies have suggested metformin may ameliorate AD-like biochemical changes [53].
No drug has yet been approved for the treatment of vascular dementia. AD treatments, such as the cholinesterase inhibitors donepezil and galantamine, have shown some cognitive benefits in clinical trials, but effects on global and functional efficacy were less consistent [54, 55]. No clear benefit with NMDA receptor antagonists (e.g., memantine) has been shown in vascular dementia. The small cognitive improvements observed in some patients with these treatments may actually result from an effect on co-existing AD [56].
Pleiotropic drugs with multimodal mechanisms of action (e.g., cerebrolysin) have shown some potential, although these findings need to be confirmed [57]. Another agent with pleiotropic neuroprotective and metabolic effects is Actovegin. The effects of Actovegin include increased oxygen utilisation and uptake, improved glucose metabolism, increased neuron survival, inhibition of poly(ADP-ribose) polymerase activity, reduced oxidative stress, activation of NF-κB, and reduced apoptosis [58]. In a randomized, double-blind, placebo-controlled trial of 567 patients, Actovegin was associated with improvements in symptoms of diabetic polyneuropathy [59]. Other studies have suggested a beneficial effect of Actovegin on cognitive function, currently being further investigated in a randomized, controlled trial [60]. Given its pleiotropic neuroprotective and metabolic actions, the effect of Actovegin on cognitive function in patients with DM may be worthy of further investigation.
Conclusions
Cognitive impairment and dementia in patients with DM is likely to become an increasing problem. However, to date, there are no specific treatments for cognitive impairment or the prevention of further cognitive decline in the general population or specifically patients with DM. Tailored, multimodal therapies may be required to reduce the risk of cognitive dysfunction and decline in patients with DM. The use of pleiotropic drugs may have a role in the treatment of cognitive dysfunction and their use may warrant further investigation in diabetic populations.
References
Gorelick PB, Scuteri A, Black SE et al (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42:2672–2713
Whiting DR, Guariguata L, Weil C et al (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94:3113–3121
Korczyn AD (2002) Mixed dementia—the most common cause of dementia. Ann N Y Acad Sci 977:129–134
Schneider JA, Bennett DA (2010) Where vascular meets neurodegenerative disease. Stroke 41(10 Suppl):S144–S146
Biessels GJ, Staekenborg S, Brunner E et al (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5:64–74
Exalto LG, Whitmer RA, Kappele LJ et al (2012) An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp Gerontol 47:858–864
Brands AM, Van den Berg E, Manschot SM et al (2007) A detailed profile of cognitive dysfunction and its relation to psychological distress in patients with type 2 diabetes mellitus. J Int Neuropsychol Soc 13:288–297
Knopman D, Boland LL, Mosley T et al (2001) Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 56:42–48
Arntzen KA, Schirmer H, Wilsgaard T et al (2011) Impact of cardiovascular risk factors on cognitive function: the Tromsø study. Eur J Neurol 18:737–743
Crane PK, Walker R, Hubbard RA et al (2013) Glucose levels and risk of dementia. N Engl J Med 369:540–548
Kerti L, Witte AV, Winkler A et al (2013) Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology 81:1746–1752
Ruis C, Biessels GJ, Gorter KJ et al (2009) Cognition in the early stage of type 2 diabetes. Diabetes Care 32:1261–1265
Yates KF, Sweat V, Yau PL et al (2012) Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol 32:2060–2067
Cheng G, Huang C, Deng H et al (2012) Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 42:484–491
Ahtiluoto S, Polvikoski T, Peltonen M et al (2010) Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 75:1195–1202
Reijmer YD, van den Berg E, Ruis C et al (2010) Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev 26:507–519
Strachan MW, R D Lawrence Lecture 2010 (2011) The brain as a target organ in type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabet Med 28:141–147
Vermeer SE, Den Heijer T, Koudstaal PJ, Rotterdam Scan Study et al (2003) Incidence and risk factors of silent brain infarcts in the population-based Rotterdam scan study. Stroke 34:392–396
Gouw AA, van der Flier WM, Fazekas F et al (2008) Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the leukoaraiosis and disability study. Stroke 39:1414–1420
Sarwar N, Gao P, Emerging Risk Factors Collaboration et al (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375:2215–2222
Eriksson M, Carlberg B, Eliasson M (2012) The disparity in long-term survival after a first stroke in patients with and without diabetes persists: the Northern Sweden MONICA study. Cerebrovasc Dis 34:153–160
Matz K, Keresztes K, Tatschl C et al (2006) Disorders of glucose metabolism in acute stroke patients: an under recognized problem. Diabetes Care 29:792–797
Brainin M, Matz K, Teuschl Y et al (2009) The hidden burden of glucose pathology in acute stroke remains hidden. Stroke 40:e3
Capes SE, Hunt D, Malmberg K et al (2001) Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32:2426–2432
Alvarez-Sabín J, Molina CA, Montaner J et al (2003) Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator-treated patients. Stroke 34:1235–1241
Bruno A, Levine SR, Frankel MR et al (2002) Admission glucose level and clinical outcomes in the NINDS rt-PA stroke trial. Neurology 59:669–674
Kruyt ND, Biessels GJ, Devries JH et al (2010) Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol 6:145–155
Uyttenboogaart M, Koch MW, Stewart RE et al (2007) Moderate hyperglycaemia is associated with favourable outcome in acute lacunar stroke. Brain 130(Pt 6):1626–1630
van den Berghe G, Wouters P, Weekers F et al (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345:1359–1367
Finfer S, Chittock DR, NICE-SUGAR Study Investigators et al (2009) Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360:1283–1297
Kansagara D, Fu R, Freeman M, Wolf F, Helfand M (2011) Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med 154:268–282
Gray CS, Hildreth AJ, Sandercock PA et al (2007) Glucose–potassium–insulin infusions in the management of post-stroke hyperglycaemia: the UK glucose insulin in stroke trial (GIST-UK). Lancet Neurol 6:397–406
Bruno A, Kent TA, Coull BM et al (2008) Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke 39:384–389
Southerland AM, Johnston KC (2012) Considering hyperglycemia and thrombolysis in the stroke hyperglycemia insulin network effort (SHINE) trial. Ann N Y Acad Sci 1268:72–78
Ligthart SA, Moll van Charante EP et al (2010) Treatment of cardiovascular risk factors to prevent cognitive decline and dementia: a systematic review. Vasc Health Risk Manag 6:775–785
Tzourio C, Anderson C, Chapman N et al (2003) Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 163:1069–1075
Peters R, Beckett N, Forette F et al (2008) Incident dementia and blood pressure lowering in the hypertension in the very elderly trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 7:683–689
Prince MJ, Bird AS, Blizard RA et al (1996) Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council’s treatment trial of hypertension in older adults. BMJ 312:801–805
Lithell H, Hansson L, Skoog I et al (2003) The study on cognition and prognosis in the elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 21:875–886
Heart Protection Study Collaborative Group (2002) MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo controlled trial. Lancet 360:7–22
Shepherd J, Blauw GJ, Murphy MB, PROspective Study of Pravastatin in the Elderly at Risk et al (2002) Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 360:1623–1630
Ryan CM, Freed MI, Rood JA et al (2006) Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care 29:345–351
Luchsinger JA, Palmas W, Teresi JA et al (2011) Improved diabetes control in the elderly delays global cognitive decline. J Nutr Health Aging 15:445–459
Jacobson AM, Musen G, Ryan CM et al (2007) Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 356:1842–1852
Koekkoek PS, Ruis C, van den Donk M et al (2012) Intensive multifactorial treatment and cognitive functioning in screen-detected type 2 diabetes-the ADDITION-Netherlands study: a cluster-randomized trial. J Neurol Sci 314:71–77
Launer LJ, Miller ME, Williamson JD et al (2011) Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 10:969–977
Whitmer RA, Karter AJ, Yaffe K et al (2009) Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 301:1565–1572
Risner ME, Saunders AM, Altman JF et al (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J 6:246–254
Harrington C, Sawchak S, Chiang C et al (2011) Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr Alzheimer Res 8:592–606
Seaquist ER, Miller ME, Fonseca V et al (2013) Effect of thiazolidinediones and insulin on cognitive outcomes in ACCORD-MIND. J Diabetes Complicat 27:485–491
Chen Y, Zhou K, Wang R et al (2009) Antidiabetic drug metformin (Glucophage) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci USA 106:3907–3912
Moore EM, Mander AG, Ames D et al (2013) Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 36:2981–2987
Li J, Deng J, Sheng W, Zuo Z (2012) Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav 101:564–574
Black S, Román GC, Geldmacher DS et al (2003) Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke 34:2323–2330
Auchus AP, Brashear HR, Salloway S et al (2007) Galantamine treatment of vascular dementia: a randomized trial. Neurology 69:448–458
Korczyn AD, Vakhapova V, Grinberg LT (2012) Vascular dementia. J Neurol Sci 322:2–10
Guekht AB, Moessler H, Novak PH et al (2011) Cerebrolysin in vascular dementia: improvement of clinical outcome in a randomized, double-blind, placebo-controlled multicenter trial. J Stroke Cerebrovasc Dis 20:310–318
Machicao F, Muresanu DF, Hundsberger H et al (2012) Pleiotropic neuroprotective and metabolic effects of Actovegin’s mode of action. J Neurol Sci 5(322):222–227
Ziegler D, Movsesyan L, Mankovsky B et al (2009) Treatment of symptomatic polyneuropathy with Actovegin in type 2 diabetic patients. Diabetes Care 32:1479–1484
Guekht A, Skoog I, Korczyn AD et al (2013) A randomised, double-blind, placebo-controlled trial of Actovegin in patients with post-stroke cognitive impairment: ARTEMIDA study design. Dement Geriatr Cogn Disord Extra 3:459–467
Acknowledgments
This review summarizes discussions of an expert meeting held in Munich, August 2012 and sponsored by Takeda Pharmaceuticals International GmbH. The sponsor provided financial support for Andy Bond of Spirit Medical Communications to draft this review and co-ordinate author review during. Authors received honoraria and travel reimbursement to attend this meeting but no honoraria were received for involvement in this paper. The final version of the paper is entirely the responsibility of the named authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bornstein, N.M., Brainin, M., Guekht, A. et al. Diabetes and the brain: issues and unmet needs. Neurol Sci 35, 995–1001 (2014). https://doi.org/10.1007/s10072-014-1797-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1797-2