Abstract
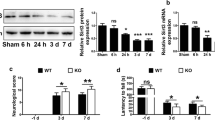
Recent studies on cerebral ischemic stroke have demonstrated the importance of the inflammatory response. Ongoing inflammatory insults have been implicated as a secondary mechanism underlying neuronal injury induced by ischemia, and anti-inflammatory strategies have gained considerable interest. Selenoprotein S (SelS), which is an endoplasmic reticulum resident protein, is known to promote cell survival by regulating inflammation. Moreover, SelS has been shown to be responsive to ischemia in cultured astrocytes. A Finnish report revealed that a variation in the SelS gene locus is associated with a higher predisposition to ischemic stroke in humans, suggesting a crucial role for SelS in protection against brain ischemia. However, the time-course of SelS expression following cerebral ischemia in vivo remains unknown. In the present study, we show, for the first time, differential SelS expression from 3 h to 7 days after reperfusion in rats with transient focal cerebral ischemia induced by a 1-h middle cerebral artery occlusion. We found that the SelS protein level decreased in the ischemic core 3–7 days after reperfusion. Furthermore, SelS expression was upregulated in the ischemic penumbra adjacent to the ischemic core 3–7 days after reperfusion and is matched by reactive astrogliosis. Thus, we propose that the upregulation of Sels represents a reaction of astrocytes against inflammatory stimuli, and the findings of this study open a new chapter in the research of the interrelationships between SelS and cerebral ischemic stroke.




Similar content being viewed by others
References
Andersson J, Johansson L, Ladenvall P, Wiklund PG, Stegmayr B, Jern C, Boman K (2009) C-reactive protein is a determinant of first-ever stroke, prospective nested case-referent study. Cerebrovasc Dis 27:544–551
Alanne M, Kristiansson K, Auro K, Silander K, Kuulasmaa K, Peltonen L, Salomaa V, Perola M (2007) Variation in the selenoprotein S gene locus is associated with coronary heart disease and ischemic stroke in two independent Finnish cohorts. Hum Genet 122:355–365
Barone FC (2009) Ischemic stroke intervention requires mixed cellular protection of the penumbra. Curr Opin Investig Drugs 10:220–223
Beamer NB, Coull BM, Clark WM, Briley DP, Wynn M, Sexton G (1998) Persistent inflammatory response in stroke survivors. Neurology 50:1722–1728
Bellinger FP, Raman AV, Reeves MA, Berry MJ (2009) Regulation and function of selenoproteins in human disease. Biochem J 422:11–22
Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, MacCluer JW, Collier GR, Kissebah AH, Blangero J (2005) Genetic variation in selenoprotein S influences inflammatory response. Nat Genet 37:1234–1241
Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ, Acute Stroke Investigators (2005) A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry 76:1366–1372
Fradejas N, Pastor MD, Mora-Lee S, Tranque P, Calvo S (2008) SEPS1 gene is activated during astrocyte ischemia and shows prominent antiapoptotic effects. J Mol Neurosci 35:259–265
Fradejas N, Serrano-Pérez Mdel C, Tranque P, Calvo S (2011) Selenoprotein S expression in reactive astrocytes following brain injury. Glia 59:59–972
Idicula TT, Brogger J, Naess H, Waje-Andreassen U, Thomassen L (2009) Admission C-reactive protein after acute ischemic stroke is associated with stroke severity and mortality, the ‘Bergen stroke study’. BMC Neurol 9:18
Kriz J (2006) Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol 18:145–157
Kuhlmann CR, Librizzi L, Closhen D, Pflanzner T, Lessmann V, Pietrzik CU, de Curtis M, Luhmann HJ (2009) Mechanisms of C-reactive protein-induced blood-brain barrier disruption. Stroke 40:1458–1466
Licata G, Tuttolomondo A, Corrao S, Di Raimondo D, Fernandez P, Caruso C, Avellone G, Pinto A (2006) Immunoinflammatory activation during the acute phase of lacunar and non-lacunar ischemic stroke: association with time of onset and diabetic state. Int J Immunopathol Pharmacol 19:639–646
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Menzies SA, Hoff JT, Betz AL (1992) Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery 31:100–107
Petito CK, Olarte JP, Roberts B, Nowak TS Jr, Pulsinelli WA (1998) Selective glial vulnerability following transient global ischemia in rat brain. J Neuropath Exp Neurol 57:231–238
Robertson J, Beaulieu JM, Doroudchi MM, Durham HD, Julien JP, Mushynski WE (2001) Apoptotic death of neurons exhibiting peripherin aggregates is mediated by the proinflammatory cytokine tumor necrosis factor-alpha. J Cell Biol 155:217–226
Rothwell NJ, Luheshi GN (2000) Interleukin 1 in the brain, biology, pathology and therapeutic target. Trends Neurosci 23:618–625
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, Licata G (2009) Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem 9:1240–1260
Tu XK, Yang WZ, Shi SS, Wang CH, Zhang GL, Ni TR, Chen CM, Wang R, Jia JW, Song QM (2010) Spatio-temporal distribution of inflammatory reaction and expression of tlr2/4 signaling pathway in rat brain following permanent focal cerebral ischemia. Neurochem Res 35:1147–1155
Vila N, Castillo J, Davalos A, Chamorro A (2000) Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 31:2325–2329
Wang Q, Tang XN, Yenari MA (2007) The inflammatory response in stroke. J Neuroimmunol 184:53–68
Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA (2004) A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429:841–847
Acknowledgments
This study was supported by Special foundation for Taishan Scholars.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L.X., Zhou, X.Y., Li, C.S. et al. Selenoprotein S expression in the rat brain following focal cerebral ischemia. Neurol Sci 34, 1671–1678 (2013). https://doi.org/10.1007/s10072-013-1319-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-013-1319-7