Abstract
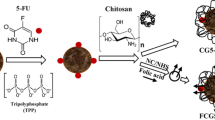
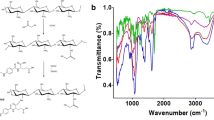
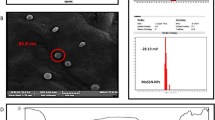
Folate conjugated chitosan coated EGCG nanoparticles (FCS-EGCG-NPs) were prepared using the ionic gelation method with folic acid modified carboxymethyl chitosan (FA-CMC) and chitosan hydrochloride as carriers of catechin EGCG. Characteristics of FCS-EGCG-NPs were determined using transmission electron microscopy (TEM) and fourier transform infrared spectroscopy (FTIR). Synthesized FCS-EGCG-NPs were spherical in shape with a mean diameter of 400 nm. The maximum encapsulation rate of nanoparticles loaded with EGCG was 75%. FTIR spectra suggested formation of an amide linkage between carboxyl groups of FA-CMC and the amine groups of chitosan hydrochloride. FCS-EGCG-NPs demonstrated sustained release of EGCG in buffer solutions of different pH values. The antitumor activity of FCS-EGCG-NPs towards different cancer cells was also investigated. FCS-EGCG-NPs had a greater tumor inhibition effect on cancer cells having a large expression of folic acid receptors on the surface than cancer cells with lesser expression.
Similar content being viewed by others
References
Sukhthankar M, Alberti S, Baek SJ. (-)-Epigallocatechin-3-gallate (EGCG) post-transcriptionally and post-translationally suppresses the cell proliferative protein TROP2 in human colorectal cancer cells. Anticancer Res. 30: 2497–2503 (2010)
Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front. Biosci. 13: 440–452 (2008)
Sen T, Chatterjee A. Epigallocatechin-3-gallate (EGCG) downregulates EGF-induced MMP-9 in breast cancer cells: Involvement of integrin receptor alpha 5 beta 1 in the process. Eur. J. Nutr. 50: 465–478 (2011)
Landis-Piwowar K, Chen D, Chan TH, Dou QP. Inhibition of catechol-O-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (-)-EGCG. Oncol. Rep. 24: 563–569 (2010)
Deng YT, Lin JK. EGCG inhibits the invasion of highly invasive CL1-5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/M arrest. J. Agr. Food Chem. 59: 13318–13327 (2011)
Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1 alpha. Carcinogenesis 32: 1881–1889 (2010)
Choudhury SR, Balasubramanian S, Chew YC, Han B, Marquez VE, Eckert RL. (-)-Epigallocatechin-3-gallate and DZNep reduce polycomb protein level via a proteasome-dependent mechanism in skin cancer cells. Carcinogenesis 32: 1525–1532 (2011)
Nandakumar V, Vaid M, Katiyar SK. (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16(INK4a), by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis 32: 537–544 (2011)
Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 25: 1198–1207 (2011)
Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 5: 14 (2010)
Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Bio. Pharm. 82: 1807–1821 (2011)
Mathew ME, Mohan JC, Manzoor K, Nair SV, Tamura H, Jayakumar R. Folate conjugated carboxymethyl chitosan-manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Carbohyd. Polym. 80: 442–448 (2010)
Sadahiro S, Suzuki T, Maeda Y, Tanaka A, Ogoshi K, Kamijo A, Murayama C, Tsukioka S, Sakamoto E, Fukui Y, Oka T. Molecular determinants of folate levels after leucovorin administration in colorectal cancer. Cancer Chemoth. Pharm. 65: 735–742 (2010)
Crane LMA, Arts HJG, van Oosten M, Low PS, van der Zee AG, van Dam GM, Bart J. The effect of chemotherapy on expression of folate receptor-alpha in ovarian cancer. Cell. Oncol. 35: 9–18 (2012)
Nunez MI, Behrens C, Woods DM, Lin H, Suraokar M, Kadara H, Hofstetter W, Kalhor N, Lee JJ, Franklin W, Stewart DJ, Wistuba II. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR mutation. Mod. Path. 24: 420–421 (2011)
Zhao LM, Shi LE, Zhang ZL, Chen JM, Shi DD, Yang J, Tang ZX. Preparation and application of chitosan nanoparticles and nanofibers. Brazilian J. Chem. Eng. 28: 353–362 (2011)
Bosselmann S, Williams RO. Has nanotechnology led to improved therapeutic outcomes? Drug Dev. Ind. Pharm. 38: 158–170 (2012)
Gong YK, Winnik FM. Strategies in biomimetic surface engineering of nanoparticles for biomedical applications. Nanoscale 4: 360–368 (2012)
Paulo CSO, Pires das Neves R, Ferreira LS. Nanoparticles for intracellular-targeted drug delivery. Nanotechnology 22: 494002 (2011)
Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 3: 16–20 (2009)
Hu B, Ting Y, Yang X, Tang W, Zeng X, Huang Q. Nanochemoprevention by encapsulation of (-)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem. Commun. 48: 2421–2423 (2012)
El-Shabouri MH. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 249: 101–108 (2002)
Hu B, Pan C, Sun Y, Hou Z, Ye H, Zeng X. Optimization of fabrication parameters to produce chitosan-tripolyphosphate nanoparticles for delivery of tea catechins. J. Agr. Food Chem. 56: 7451–7458 (2008)
Sayin B, Somavarapu S, Li XW, Thanou M, Sesardic D, Alpar HO, Senel S. Mono-N-carboxymethyl chitosan (MCC) and N-trimethyl chitosan (TMC) nanoparticles for non-invasive vaccine delivery. Int. J. Pharm. 363: 139–148 (2008)
Liang J, Li F, Fang Y, Yang WJ, An XX, Zhao LY, Xin ZH, Hu QH. Response surface methodology in the optimization of tea polyphenols-loaded chitosan nanoclusters formulations. Eur. Food Res. Tech. 231: 917–924 (2010)
Liang J, Li F, Fang Y, Yang WJ, An XX, Zhao LY, Xin ZH, Cao L, Hu QH. Synthesis, characterization and cytotoxicity studies of chitosan-coated tea polyphenols nanoparticles. Colloid. Surface. B 82: 297–301 (2011)
Dehshahri S, Wink M, Afsharypuor S, Asghari G, Mohagheghzadeh A. Antioxidant activity of methanolic leaf extract of Moringa peregrina (Forssk.) Fiori. Res. Pharm. Sci. 2: 111–118 (2012)
Li P, Wang Y, Zeng F, Chen L, Peng Z, Kong LX. Synthesis and characterization of folate conjugated chitosan and cellular uptake of its nanoparticles in HT-29 cells. Carbohyd. Res. 346: 801–806 (2011)
Kosaraju SL, D’Ath L, Lawrence A. Preparation and characterisation of chitosan microspheres for antioxidant delivery. Carbohyd. Polym. 64: 163–167 (2006)
Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur. J. Pharm. Sci. 41: 219–225 (2010)
Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the plasma exposure of (-)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 44: 422–426 (2011)
Galbiati A, Tabolacci C, Morozzo Della Rocca B, Mattioli P, Beninati S, Paradossi G, Desideri A. Targeting tumor cells through chitosan-folate modified microcapsules loaded with camptothecin. Bioconjug. Chem. 22: 1066–1072 (2011)
Gong JL, Wang SM, Hu XG, Cao MM, Zhang JR. Synthesis and characterization of folic acid-conjugated chitosan nanoparticles as a tumor-targeted drug carrier. Nan Fang Yi Ke Da Xue Xue Bao (China) 28: 2183–2186 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, J., Cao, L., Zhang, L. et al. Preparation, characterization, and in vitro antitumor activity of folate conjugated chitosan coated EGCG nanoparticles. Food Sci Biotechnol 23, 569–575 (2014). https://doi.org/10.1007/s10068-014-0078-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-014-0078-4