Abstract
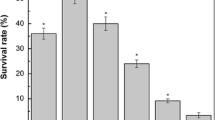
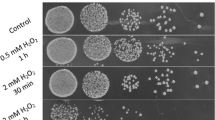
Leuconostoc mesenteroides is a commercially important lactic acid bacterium which is used as a starter culture in various fermentation processes. However, because of its sensitivity to acid stress, high cell-density culture has not been accomplished yet. Therefore, we investigated the effect of preadaptation of L. mesenteroides under mildly acidic conditions for resistance to normally lethal levels of acid stress. For this, the cells grown to the early-exponential phase at pH 7.0 were incubated at pH 6.5, 6.0, and 5.0 for 1 h, and then the survival rates of acid-adapted cells in pH 4.0 solution was determined. Acid-adapted cells at pH 5.0 exhibited maximum increase in tolerance, showing an increased survival of approximately 2,500 folds compared to the control (pH 7.0).
Similar content being viewed by others
References
Eom HJ, Park JM, Seo MJ, Kim MD, Han NS. Mornitoring of Leuconostoc mesenteroides DRC starter in fermented vegetable by random integration of chloramphenicol acetyltransferase. J. Ind. Microbiol. Biotechnol. 35: 953–959 (2008)
Kristek S, Bešlo D, Pavloviæ H, Kristek A. Effect of starter cultures L. mesenteroides and L. lactis spp. lactis on sauerkraut fermentation and quality. Czech J. Food Sci. 22: 125–132 (2004)
Kaletunc G, Lee J, Alpas H, Bozoglu F. Evaluation of structural changes induced by high hydrostatic pressure in Leuconostoc mesenteroides. Appl. Environ. Microbiol. 70: 1116–1122 (2004)
McDonald LC, Fleming HP, Hassan HM. Acid tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl. Environ. Microbiol. 56: 2120–2124 (1990)
Kobayashi H, Murakami N, Unemoto T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J. Biol. Chem. 257: 13246–13252 (1982)
Herrero AA. End-product inhibition in anaerobic fermentations. Trends Biotechnol. 1: 49–53 (1983)
O’Sullivan E, Condon S. Intracellular pH is a major factor in the induction of tolerance to acid and other stresses in Lactococcus lactis. Appl. Environ. Microbiol. 63: 4210–4215 (1997)
Rallu F, Gruss A, Ehrlich SD, Maguin E. Acid- and multistressresistant mutants of Lactococcus lactis: Identification of intracellular stress signals. Mol. Microbiol. 35: 517–528 (2000)
Slonczewski JL, Foster JW. pH-Regulated genes and survival at extreme pH. Vol. 1, pp. 1539–1549. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds). 2nd ed. ASM Press, Washington, DC, USA (1996)
Foster JW. Low pH adaptation and the acid tolerance response of S. typhimurium. Crit. Rev. Microbiol. 21: 215–237 (1995)
Minton KW, Karmin P, Hahn GM, Minton AP. Nonspecific stabilization of stress-susceptible proteins by stress resistant proteins: A model for the biological role of heat shock proteins. P. Natl. Acad. Sci. USA 79: 7107–7111 (1982)
Budin-Verneuil A, Pichereau V, Auffray Y, Ehrlich DS, Maguin E. Proteomic characterization of the acid tolerance response in Lactococcus lactis MG1363. Proteomics 5: 4794–4807 (2005)
Kwon HY, Kim SW, Choi MH, Ogunniyi AD, Paton JC, Park SH, Pyo SN, Rhee DK. Effect of heat shock and mutations in ClpL and ClpP on virulence gene expression in Streptococcus pneumonia. Infect. Immun. 71: 3757–3765 (2003)
Lorca GL, Valdez GF. A low-pH-inducible, stationary-phase acid tolerance response in Lactobacillus acidophilus CRL 639. Curr. Microbiol. 42: 21–25 (2001)
Len AC, Harty DW, Jacques NA. Stress-responsive proteins as upregulated in Streptococcus mutans during acid tolerance. Microbiology 150: 1339–1351 (2004)
Martín-Galiano AJ, Overweg K, Ferrándiz MJ, Reuter M, Wells JM, de la Campa AG. Transcritptional analysis of the acid tolerance response in Streptococcus pneumonia. Microbiology 151: 3935–3946 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.E., Eom, HJ., Li, L. et al. Induction of the acid tolerance response in Leuconostoc mesenteroides ATCC 8293 by pre-adaptation in acidic condition. Food Sci Biotechnol 23, 221–224 (2014). https://doi.org/10.1007/s10068-014-0030-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-014-0030-7