Abstract
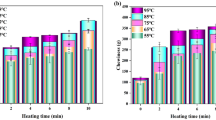
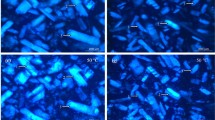
The texture and microstructure of edible abalone meats were studied during heat treatments from 50 to 100°C for 60 min. No increase in extractable soluble collagen content was observed below 80°C, but a 9-fold increase was observed at 100°C. SDS-PAGE showed that extractable myosin heavy chains and paramyosin contents reduced significantly at 80°C, and disappeared completely at 100°C. The shear force increased slowly from 50 to 70°C, but relaxed back to the initial level at 100°C. Rapid reduction of hardness was observed at 50°C, minimum hardness was obtained at 100°C. Springness, cohesiveness, chewiness, and resilience were enhanced to maximum levels at 70, 90, 70, and 90°C, respectively. Optical micrographs and transmission electron microscope showed a significant increase of intermyofibrillar gaps at 90°C and broken fibers at 100°C. Results suggested that 80°C might be a suitable temperature to produce ready-to-eat abalone products.
Similar content being viewed by others
References
Olley J, Thrower SJ. Abalone — An esoteric food. Adv. Food Res. 23: 143–186 (1977)
Oakes FR, Ponte RD. The abalone market: Opportunities for cultured abalone. Aquaculture 140: 187–195 (1996)
Olaechea RP, Ushio H, Watabe S, Takada K, Hatae K. Toughness and collagen content of abalone muscles. Biosci. Biotech. Bioch. 57: 6–11 (1993)
Wang YY, Zhu BW, Dong XP, Ye WX, Xiao GH. Constituent and characterization of myofibrillar protein from abalone. p.154. In: Research on Theory and Technology of Precious Seafood Processing. Beiwei Zhu (ed). Science Press, Beijing, China (2010)
Ehara T, Nakagawa K, Tamiya T, Noguchi SF, Tsuchiya T. Effect of paramyosin on invertebrate natural actomyosin gel formation. Fisheries Sci. 70: 306–313 (2004)
Gao X, Ogawa H, Tashiro Y, Iso N. Rheological properties and structural changes in raw and cooked abalone meat. Fisheries Sci. 67: 314–320 (2001)
Califano AN, Bertola NC, Bevilacqua AE, Zaritzky NE. Effect of processing conditions on the hardness of cooked beef. J. Food Eng. 34: 41–54 (1997)
Palka K, Daun H. Changes in texture, cooking losses, and myofibrillar structure of bovine M. semitendinosus during heating. Meat Sci. 51: 237–243 (1999)
García-Segovia P, Andrés-Bello A, Martínez-Monzó J. Effect of cooking method on mechanical properties, color, and structure of beef muscle (M. pectoralis). J. Food Eng. 80: 813–821 (2007)
Harris PV, Shorthose WR. Meat texture. pp. 245–296. In: Developments in Meat Science — 4. Lawrie RA (ed). Elsevier Applied Science Publisher, London, UK (1988)
Pascual M, Pla M. Changes in collagen, texture, and sensory properties of meat when selecting rabbits for growth rate. Meat Sci. 78: 375–380 (2008)
Eilert SJ, Mandigo RW. Procedure for soluble collagen in thermally processed meat products. J. Food Sci. 58: 948–949 (1993)
Hatae K, Nakai H, Tanaka C, Shimada A, Watabe S. Taste and texture of abalone meat after extended cooking. J. Food Sci. 64: 643–647 (1996)
Gao X, Tashiro Y, Ogawa H. Rheological properties and structural changes in steamed and boiled abalone meat. Fisheries Sci. 68: 499–508 (2002)
Gao X, Zhang ZH, Tang ZX, Yuri T, Hiroo O. The relationships between rheological properties and structural changes of chilled abalone meat. J. Ocean Univ. China 2: 171–176 (2003)
Chiou TK, Tsai CY, Lan HL. Chemical, physical, and sensory changes of small abalone meat during cooking. Fisheries Sci. 70: 867–874 (2004)
Wattanachant S, Benjakul S, Ledward DA. Effect of heat treatment on changes in texture, structure, and properties of Thai indigenous chicken muscle. Food Chem. 93: 337–348 (2005)
Vaithiyanathan S, Naveena BM, Muthukumar M, Girish PS, Ramakrishna C, Sen AR, Babji Y. Biochemical and physicochemical changes in spent hen breast meat during postmortem aging. Poultry Sci. 87: 180–186 (2008)
Kimura S, Kubota M. Some properties of collagen from the abalone. Nippon Suisan Gakk. 34: 925–929 (1968)
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970)
Bourne MC. Texture profile analysis. Food Technol.-Chicago 32: 62–66 (1978)
Liu SX. Olefin cutting, common dyeing methods. pp. 55–73, 133–149. In: Practical Histological Technology. Liu SX (ed). Science Press, Beijing, China (2004)
Chan JK, Gill TA, Paulson AT. Cross-linking of myosin heavy chains from cod, herring, and silver hake during thermal setting. J. Food Sci. 57: 906–912 (1992)
Ko WC, Yu CC, Hsu KC. Changes in conformation and sulfhydryl groups of tilapia actomyosin by thermal treatment. LWT-Food Sci. Technol. 40: 1316–1320 (2007)
Watabe S, Hartshorne DJ. Paramyosin and the catch mechanism. Comp. Biochem. Phys. B 96: 639–646 (1990)
Suzuki M, Kobayashi Y, Hiraki Y, Nakata H, Shiomi K. Paramyosin of the disc abalone Haliotis discus: Identification as a new allergen and cross-reactivity with tropomyosin. Food Chem. 124: 921–926 (2011)
Lin WL, Zeng QX, Zhu ZW. Different changes in mastication between crisp grass carp (Ctenopharyngodon idellus C.et V) and grass carp (Ctenopharyngodon idellus) after heating: The relationship between texture and ultrastructure in muscle tissue. Food Res. Int. 42: 271–278 (2009)
Ayala MD, Albors OL, Blanco A, Alcázar AG, Abellán E, Zarzosa GR, Gil F. Structural and ultrastructural changes on muscle tissue of sea bass, Dicentrarchus labrax L., after cooking and freezing. Aquaculture 250: 215–231 (2005)
Kong FB, Tang JM, Lin MS, Rasco B. Thermal effects on chicken and salmon muscles: Tenderness, cook loss, area shrinkage, collagen solubility, and microstructure. LWT-Food Sci. Technol. 41: 1210–1222 (2008)
Kong F, Tang J, Rasco B, Crapo C, Smiley S. Quality changes of salmon (Oncorhynchus gorbuscha) muscle during thermal processing. J. Food Sci. 72: 103–111 (2007)
Hatae K, Nakai H, Shimada A, Murakami T, Takada K, Shirojo Y, Watabe S. Abalone (Hariltis discus): Seasonal variations in chemical composition and textural properties. J. Food Sci. 60: 32–35 (1995)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, B., Dong, X., Sun, L. et al. Effect of thermal treatment on the texture and microstructure of abalone muscle (Haliotis discus). Food Sci Biotechnol 20, 1467–1473 (2011). https://doi.org/10.1007/s10068-011-0203-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-011-0203-6