Abstract
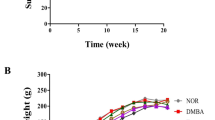
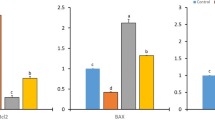
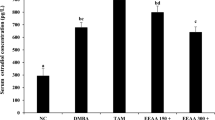
This study was to demonstrate whether loquat leaves protect against 7,12-dimethylbenz[α]anthracene (DMBA)-induced breast cancer in female rats. Rats were fed control diet (NC, DC), ethanol (DE), or water extract of loquat leaves (DW) until sacrifice. At 50 days of age, animals in the DC, DE, and DW groups were given a single intra-gastric dose of DMBA (50 mg/kg) dissolved in corn oil. Both the water and ethanol extracts of loquat leaves decreased the incidence of tumors, with water extract having the higher inhibitory effect. Ethanol extract significantly decreased the multiplicity of tumors. Loquat extracts significantly decreased tumor cell proliferation and the tumor grade based on the degree of tubule formation. These results suggest that both water and ethanol extracts of loquat leaves inhibit the development of breast cancer. Such inhibition may be based on the suppression of initiation and tumor cell proliferation.
Similar content being viewed by others
References
Qin LQ, Xu JY, Wang PY, Ganmaa D, Li J, Wang J, Kaneko T, Hoshi K, Shirai T, Sata A. Low-fat milk promotes the development of 7,12-dimethylbenz(a)-anthracene-induced mammary tumors in rats. Int. J. Cancer 110: 491–496 (2004)
Heimann R, Jeuson JC, Coune A. Tumors developing in oophorectomized Sprague-Dawley rats after a single gastric instillation of 7,12-dimethylbenz(a)-anthracene. Cancer Res. 28: 309–313 (1968)
Willett WC. Diet and breast cancer. J. Int. Med. 249: 395–411 (2001)
Mehta RG, Pezzuto JM. Phytochemicals as potential cancer chemopreventive agents. pp. 237–246. In: Phytopharmaceuticals in Cancer Chemoprevention. Bagchi D, Preuss HG (eds). CRC Press, Boca Raton, FL, USA (2005)
Ray SD, Bagchi D. Roles of polyphenols, flavonoids, and oligomeric proanthocyanidins in cancer prevention. pp. 311–345. In: Phytopharmaceuticals in Cancer Chemoprevention. Bagchi D, Preuss HG (eds). CRC Press, Boca Raton, FL, USA (2005)
Prior RL, Joseph J. Berries and fruits in cancer chemoprevention. pp. 465–480. In: Phytopharmaceuticals in Cancer Chemoprevention. Bagchi D, Preuss HG (eds). CRC Press, Boca Raton, FL, USA (2005)
Kim MS, You MK, Rhuy DY, Kim YJ, Baek HY, Kim HA. Loquat (Eriobotrya japonica) extracts suppress the adhesion, migration, and invasion of human breast cancer cell line. Nutr. Res. Pract. 3: 259–264 (2009)
Banno N, Akihisa T, Tokuda H, Yasukawa K, Taguchi Y, Akazawa H, Ukiya M, Kinura Y, Suxuki T, Nishino H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol. Pharm. Bull. 25: 1995–1999 (2005)
Kim E, Kin MS, Rhuy DY, Min OJ, Baek HY, Kim YJ, Kim HA. Hypoglycemic effect of Eryobotrya japonica in db/db mice. Korean J. Food Nutr. 22: 159–165 (2009)
Koba K, Matsuoka A, Osada K, Huang YS. Effect of loquat (Eriobotrya japonica) extracts on LDL oxidation. Food Chem. 104: 308–316 (2007)
Ito H, Kobayashi E, Li SH, Hatano T, Sugita D, Kuvo N, Shimura S, Itoh Y, Tokuda H, Nishino H, Yoshida T. Antitumor activity of compounds isolated from leaves of Eriobotrya japonica. J. Agr. Food Chem. 52: 2400–2403 (2002)
Taniguchi S, Imayoshi Y, Kobayashi E, Takamatsu Y, Ito H, Hatani T, Sakagami H, Tokuda H, Nishino H, Daigo S, Shimura S, Yoshida T. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry 59: 315–323 (2002)
Kwon HJ, Kang MJ, Kin HJ, Choi JS, Paik KJ, Chung HY. Inhibition of NFκB by methyl chlorogenate from Eriobotrya japonica. Mol. Cell 10: 241–246 (2000)
Ito H, Kobayashi E, Takamatsu Y, Li S, Hatano T, Sakagami H, Kusama K, Satoh K, Sugita D, Shimura S, Itoh Y, Yoshida T. Polyphenols from Eribotrya japonica and their cytotoxicity against human oral cancer cell lines. Chem. Pharm. Bull. 48: 687–693 (2000)
Komiya T, Achiwa Y, Katsuzaki H, Sakurai S, Urakawa K, Ohnish K, Adachi T, Yanada T, Hibasami H. Effect of oleanolic and ursolic acids from loquat (Eriobotrya japonica) on the growth of human lymphoid leukemia cells. Food Sci. Technol. Int. 4: 282–284 (1998)
Tokuda H, Ohigashi H, Kishimizu K, Ito Y. Inhibitory effects of ursolic and oleanolic acids on skin tumor promotion by 12-Otetradecanoylphorbol-13-acetate. Cancer Lett. 33: 279–285 (1986)
Zheng S, Sandeep S, Ning L, Divya R, Peiying Y, Robert AN, Chung SY, Xiaoxin C. Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7,12-dimethylbenz[a] anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch, and inhibition of carcinogenesis by its inhibitors. Carcinogenesis 27: 1902–1908 (2006)
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: Experience from a large study with long-term follow up. Histopathology 19: 403–410 (1991)
Samy RP, Fopalakrishnakone P, Ignacimuthu S. Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chem. Biol. Interact. 164: 1–14 (2006)
Mehta RG. Experimental basis for the prevention of breast cancer. Eur. J. Cancer 36: 1275–1282 (2000)
Takahashi M, Shibutani GH, Woo K, Inoue H, Fujimoto K, Igarashi J, Kanno M, Hirose, Nishikawa A. Cellular distributions of molecules with altered expression specific to the tumor promotion process from the early stage in a rat two-stage hepatocarcinogenesis model. Carcinogenesis 29: 2218–2226 (2008)
Fritz WS, Coward L, Wang J, Lamartiniere C. Dietary genestein: Perinatal mammary cancer prevention, bioavailability, and toxicity testing in the rat. Carcinogenesis 19: 2151–2158 (1998)
Li N, Chen X, Liao J, Yang G, Wang S, Josephson Y, Han C, Chen J, Huang MT, Yang CS. Inhibition of 7,12-dimethylbenz[a] anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 23: 1307–1313 (2002)
Reeves PG, Nielsen FH, Fahey GC. AIN-93 puried diets for laboratory rodents: Final report of the American Institute of Nutrition adhoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123: 1939–1951 (1993)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M.S., You, M.K., Rhyu, D.Y. et al. Oral administration of loquat suppresses DMBA-induced breast cancer in rats. Food Sci Biotechnol 20, 491–497 (2011). https://doi.org/10.1007/s10068-011-0068-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-011-0068-8