Abstract
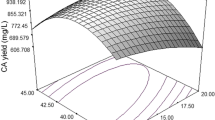
Present study reports statistical media optimization for dextran production for lactic acid bacteria, isolated from Indian traditional fermented idli batter. Morphological, biochemical, and 16S rRNA sequencing analysis identified the strain as Leuconostoc mesenteroides. A sequential statistical methodology comprising of Plackett-Burman and response surface methodology (RSM) were applied to enhance the fermentative production of dextran. A quadratic polynomial equation suggested by the RSM model was then validated experientially. The predicted yield by model was 60.73 g/L. Experimental verification of the model resulted in 60.30 g/L dextran. The experimental values were found to be very close to the predicted values and hence, the model was successfully validated. Simple basal medium gave 7.83 g/L dextran. Thus dextran production was increased by 7.70 fold over the unoptimized basal medium using these statistical techniques. Structural characteristic of the dextran were determined by Fourier transform infrared, 1H and 13C nuclear magnetic resonance spectroscopic techniques.
Similar content being viewed by others
References
Tannock GW. A special fondness for lactobacilli. Appl. Environ. Microb. 70: 3189–3194 (2004)
Sandhu DK, Soni SK. Indian fermented foods: Microbiological and biochemical aspects. Indian J. Microbiol. 30: 135–157 (1990)
Nagaoka M, Hashimito S, Watanabe T, Yokokura T, Mori T. Antiulcer effects of lactic acid bacteria and their cell wall polysaccharides. Biol. Pharm. Bull. 17: 1012–1017 (1994)
Nakajima H, Suzuki Y, Kaizu H, Hirota T. Cholesterol lowering activity of ropy fermented milk. J. Food Sci. 57: 1327–1329 (1992)
Liu C, Lin Q, Gaom Y, Yem L, Xing Y, Xi T. Characterization and antitumor activity of polysaccharide from Strongylocentrotus nudus eggs. Carbohyd. Polym. 67: 313–318 (2007)
Sutherland IW. Novel and established applications of bacterial polysaccharides. Trends Biotechnol. 16: 41–46 (1998)
Leathers TD. Dextran. Vol. 5, pp. 299–321. In: Biopolymers. Vandamme EJ, De BS, Steinbuchel A (eds). Wiley-VCH, Weinheim, Germany (2002)
Kim D, Robyt JF, Lee SY, Lee JH, Kim YM. Dextran molecular size and degree of branching as a function of sucrose concentration, pH, and temperature of reaction of Leuconostoc mesenteroides B-512FMCM dextransucrase. Carbohyd. Res. 338: 1183–1189 (2003)
Behravan J, Bazzaz BSF, Salimi Z. Optimization of dextran production by Leuconostoc mesenteroides NRRL B-512 using cheap and local sources of carbohydrates and nitrogen. Biotechnol. Appl. Bioc. 38: 267–269 (2003)
Kartikeyan RS, Rakshit SK, Baradarajan A. Optimization of batch fermentation conditions for dextran production. Bioprocess. Eng. 15: 247–251 (1996)
Qader SAU, Iqbal L, Aman A, Shireen E, Azhar A. Production of dextran by newly isolated strain of Leuconostoc mesenteroides PCSIR-4 and PCSIR-9. Turk. J. Biochem. 31: 21–26 (2005)
Sawale SD, Lele SS. Enhanced production of dextransucrase by L. mesenteroides MTCC 867 using response surface methodology. Rec. Res. Sci. Tech. 1: 94–99 (2009)
Sawale SD, Lele SS. Increased dextransucrase production by response surface methodology from Leuconostoc species; isolated from fermented idli batter. Global J. Biochem. Biotechnol. 4: 160–167 (2009)
Vedyashkina TA, Revin VV, Gogotov IN. Optimizing the conditions of dextran synthesis by the bacterium Leuconostoc mesenteroides grown in a molasses containing medium. Appl. Biochem. Microbiol. 41: 361–364 (2005)
Shamala TR, Prasad MS. Preliminary studies on the production of high and low viscosity dextran by Leuconostoc spp. Process Biochem. 30: 237–241 (1995)
Uzochukwu S, Balogh E, Loefler RT, Ngoddy PO. Structural analysis by 13C nuclear magnetic resonance spectroscopy of glucan extracted from natural palm wine. Food Chem. 76: 287–291 (2002)
Kralj S, Van Geel-Schutten GH, Dondroff MG, Kirsanovs S, Van Der Maarel MJEC, Dijkhuizen L. Glucan synthesis in the genus Lactobacillus: Isolation and characterization of glucansucrase genes, enzymes, and glucan products from six different strains. Microbiology 150: 3681–3690 (2004)
Kalil SJ, Maugeri F, Rodrigues MI. Response surface analysis and simulation as a tool for bioprocess design and optimization. Process Biochem. 35: 539–550 (2000)
Lee JH, Chae M, Choi GH, Lee N, Paik HD. Optimization of medium composition for production of the antioxidant substances by Bacillus polyfermenticus SCD using response surface methodology. Food Sci. Biotechnol. 18: 959–964 (2009)
Tsapatsaris S, Kotzekidou P. Application of central composite design and response surface methodology to the fermentation of olive juice by Lactobacillus plantarum and Debaryomyces hansenii. Int. J. Food Microbiol. 95: 157–168 (2004)
Shabazian N, Ashriani FZ, Bonakdarpour B. Statistical optimization of the medium components for the production of protopectinases by Bacillus subtilis. Food Sci. Biotechnol. 18: 442–448 (2009)
Welman AD, Maddox IS. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnol. 21: 269–274 (2003)
Sanni AI, Onilude AA, Ogunbanwo ST, Fadahunsi IF, Afolabi RO. Production of exopolysaccharides by lactic acid bacteria isolated from traditional fermented foods in Nigeria. Eur. Food Res. Technol. 214: 405–407 (2002)
Kebede A. Isolation, characterization, and identification of lactic acid bacteria involved in traditional fermentation of borde, an Ethipian cereal beverage. Afr. J. Biotechnol. 6: 1469–1478 (2007)
Chen YS, Yangagida F, Hsu JS. Isolation and characterization of lactic acid bacteria from suan-tsai (fermented mustard), a traditional fermented food in Taiwan. J. Appl. Microbiol. 101: 125–130 (2006)
Desai KM, Akolkar SK, Badhe YP, Tambe SS, Lele SS. Optimization of fermentation media for expolysaccharide production from Lactobacillus plantarum using artificial intelligence-based techniques. Process Biochem. 41: 1842–1848 (2006)
Kim Y, Kim JU, Oh S, Kim YJ, Kim M, Kim SH. Technical optimization of culture conditions for the production of exopolysaccharides (EPS) by Lactobacillus rhamnosus ATCC 9595. Food Sci. Biotechnol. 17: 587–593 (2008)
Jung SW, Kim WJ, Lee KG, Kim CW, Noh WS. Isolation and identification of lactic acid bacteria from sourdough with high exopolysaccharide production ability. Food Sci. Biotechnol. 18: 384–389 (2009)
deMan JC, Rogosa M, Sharpe ME. A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 23: 130–135 (1961)
Garvie EI. Genus Leuconostoc. Vol. 2, pp. 1071–1075. In: Bergey’s Manual of Systematic Bacteriology. Van T, Sneath PHA, Mair NS, Sharpe ME, Holt GJ (eds). Williams and Wilkins, Baltimore, MD, USA (1986)
Plackett RL, Burman JP. The design of optimum multifactorial experiments. Biometrika 33: 305–325 (1946)
Sutherland IW. Extracellular polysaccharide. Vol 3, pp. 531–568. In: Biotechnology. Rehm HJ, Reed G (eds). Verlag Chemie, Weinheim, Germany (1983)
Tsuchiya HM, Koepsell HJ, Corman J, Bryant G, Bogard MO, Feger VH, Jackson RW. The effect of certain cultural factors on production of dextransucrase by Leuconostoc mesenteroides. J. Bacteriol. 64: 521–526 (1952)
Chasco MTD, Carvajal MAR, Mateo PT, Espartero JL, Iribas AI, d Serrano AMG. Structural analysis of the exopolysaccharides produced by Lactobacillius spp G-77. Carbohyd. Res. 307: 125–133 (1998)
Heyn ANJ. The infrared absorption spectrum of dextran and its bound water. Biopolymers 13: 475–506 (1974)
Shingel KI. Determination of structural peculiarities of dextran, pullulan, and γ-irradiated pullulan by Fourier-transform IR spectroscopy. Carbohyd. Res. 337: 1445–1451 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawale, S.D., Lele, S.S. Statistical optimization of media for dextran production by Leuconostoc sp., isolated from fermented idli batter. Food Sci Biotechnol 19, 471–478 (2010). https://doi.org/10.1007/s10068-010-0066-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-010-0066-2