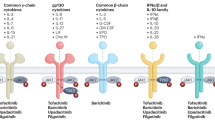
Abstract
Considerable controversy related to the cardiovascular safety of Janus kinase inhibitors (JAKinibs) has arisen following the results of the ORAL Surveillance trial. In this trial of rheumatoid arthritis (RA) ≥ 50 years with at least one prevalent cardiovascular disease (CVD) risk factor, tofacitinib was not found to be non-inferior to tumour necrosis factor-alpha inhibitors (TNFi) with regards to the risk for major adverse cardiovascular events (MACE), venous thromboembolism (VTE) or malignancy. Following the results of ORAL Surveillance, the United States Food and Drug Administration (US FDA) issued a boxed warning regarding increased risks of MACE, VTE and malignancy with tofacitinib, baricitinib or upadacitinib in inflammatory arthritis or ulcerative colitis. Analysis of data from other trials (including long-term follow-up studies) of tofacitinib in RA, psoriasis, psoriatic arthritis, spondyloarthritis and inflammatory bowel diseases suggests an overall similar risk of MACE or VTE with tofacitinib when compared with TNFi. In specific patient populations with risk factors for or prior history of MACE or VTE, the risk of subsequent MACE or VTE with tofacitinib use is considerably heightened. Post-hoc analyses from ORAL Surveillance presented at the recent EULAR meeting further help to delineate patients with RA at increased risk of MACE/VTE with tofacitinib. Based on the available literature from trials and long-term follow-up studies of baricitinib and upadacitinib, there exists insufficient evidence to extend the warning of MACE/VTE with tofacitinib to these drugs. Ongoing post-marketing surveillance studies of JAKinibs in immune-mediated inflammatory diseases should help clarify CVD risk with JAKinibs.
Key Points • The ORAL Surveillance trial failed to demonstrate non-inferiority regarding CVD and malignancy risk for tofacitinib versus TNFi, in rheumatoid arthritis ≥ 50 years old with prevalent CVD risk factors. • Information from various other trials and their long-term extensions suggest similar risk for MACE or VTE with tofacitinib and TNFi in overall patients with RA; heightened MACE/VTE risk likely applies to specific patient populations. • Post-hoc analyses of ORAL Surveillance suggest a heightened risk for MACE and VTE with tofacitinib in older RA patients with prior MACE/VTE events or risk factors. • Trials of baricitinib and upadacitinib do not appear to reveal an increased risk of CVD or VTE with these drugs at their usual doses than with TNFi or placebo; ongoing post-marketing surveillance studies might further clarify CVD/VTE risk with these drugs. |


Similar content being viewed by others
Data availability
Data pertaining to the article shall be shared on reasonable request to the corresponding author (Durga Prasanna Misra, durgapmisra@gmail.com).
References
Maini RN, Breedveld FC, Kalden JR et al (1998) Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 41:1552–1563. https://doi.org/10.1002/1529-0131(199809)41:9%3c1552::Aid-art5%3e3.0.Co;2-w
Jones G, Sebba A, Gu J et al (2010) Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 69:88–96. https://doi.org/10.1136/ard.2008.105197
Choy EH (2018) Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology (Oxford) 58:953–962. https://doi.org/10.1093/rheumatology/key339
van Vollenhoven RF, Fleischmann R, Cohen S et al (2012) Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 367:508–519. https://doi.org/10.1056/NEJMoa1112072
van der Heijde D, Deodhar A, Wei JC et al (2017) Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 76:1340–1347. https://doi.org/10.1136/annrheumdis-2016-210322
Mease P, Hall S, FitzGerald O et al (2017) Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 377:1537–1550. https://doi.org/10.1056/NEJMoa1615975
Sandborn WJ, Ghosh S, Panes J et al (2012) Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 367:616–624. https://doi.org/10.1056/NEJMoa1112168
Moriana C, Moulinet T, Jaussaud R, Decker P (2022) JAK inhibitors and systemic sclerosis: a systematic review of the literature. Autoimmun Rev 21:103168. https://doi.org/10.1016/j.autrev.2022.103168
Hasni SA, Gupta S, Davis M et al (2021) Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat Commun 12:3391. https://doi.org/10.1038/s41467-021-23361-z
Liu Y, Ji Z, Yu W et al (2021) Tofacitinib for the treatment of antineutrophil cytoplasm antibody-associated vasculitis: a pilot study. Ann Rheum Dis 80:1631–1633. https://doi.org/10.1136/annrheumdis-2021-220484
Rathore U, Thakare DR, Patro P, Agarwal V, Sharma A, Misra DP (2022) A systematic review of clinical and preclinical evidences for Janus kinase inhibitors in large vessel vasculitis. Clin Rheumatol 41:33–44. https://doi.org/10.1007/s10067-021-05973-4
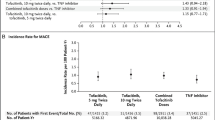
Ytterberg SR, Bhatt DL, Mikuls TR et al (2022) Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 386:316–326. https://doi.org/10.1056/NEJMoa2109927
US FDA warning on 1st September 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death. Accessed 4 Aug 2022
Delong C, Preuss CV (2022) Black box warning. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright ©, StatPearls Publishing LLC.; 2022.
EMA starts review of Janus kinase inhibitor safety. https://www.ema.europa.eu/en/documents/referral/janus-kinase-inhibitors-jaki-article-20-referral-review-started_en.pdf. Accessed 4 Aug 2022
Prescribing information for tofacitinib from the US FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf. Accessed 14 Aug 2022
US FDA warning on 25th February (2019) https://www.fda.gov/drugs/drug-safety-and-availability/safety-trial-finds-risk-blood-clots-lungs-and-death-higher-dose-tofacitinib-xeljanz-xeljanz-xr. Accessed 4 Aug 2022
US FDA warning on 26th July (2019) https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and. Accessed 4 Aug 2022
Pfizer announces results of ORAL Surveillance trial. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-co-primary-endpoint-results-post-marketing. Accessed 5 Aug 2022
US FDA warning on 4th February (2021) https://www.fda.gov/drugs/drug-safety-and-availability/initial-safety-trial-results-find-increased-risk-serious-heart-related-problems-and-cancer-arthritis. Accessed 4 Aug 2022
Charles-Schoeman C, Wicker P, Gonzalez-Gay MA et al (2016) Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum 46:261–271. https://doi.org/10.1016/j.semarthrit.2016.05.014
Charles-Schoeman C, DeMasi R, Valdez H et al (2019) Risk factors for major adverse cardiovascular events in phase III and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol 71:1450–1459. https://doi.org/10.1002/art.40911
Mease P, Charles-Schoeman C, Cohen S et al (2020) Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 79:1400–1413. https://doi.org/10.1136/annrheumdis-2019-216761
Kremer JM, Bingham CO 3rd, Cappelli LC et al (2021) Postapproval comparative safety study of tofacitinib and biological disease-modifying antirheumatic drugs: 5-year results from a United States-based rheumatoid arthritis registry. ACR Open Rheumatol 3:173–184. https://doi.org/10.1002/acr2.11232
Desai RJ, Pawar A, Khosrow-Khavar F, Weinblatt ME, Kim SC (2021) Risk of venous thromboembolism associated with tofacitinib in patients with rheumatoid arthritis: a population-based cohort study. Rheumatology (Oxford) 61:121–130. https://doi.org/10.1093/rheumatology/keab294
Khosrow-Khavar F, Kim SC, Lee H, Lee SB, Desai RJ (2022) Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis 81:798–804. https://doi.org/10.1136/annrheumdis-2021-221915
Fang YF, Liu JR, Chang SH, Kuo CF, See LC (2022) Comparative safety of Janus kinase inhibitors and tumor necrosis factor inhibitors in patients undergoing treatment for rheumatoid arthritis. Int J Rheum Dis. Epub ahead of print. https://doi.org/10.1111/1756-185x.14414
Bilgin E, Duran E, Ünaldı E, Kalyoncu U, Kiraz S, Ertenli İ (2022) Comparison of cardiovascular, cancer and herpes zoster risk of tofacitinib versus etanercept: single centre observational study. Rheumatology (Oxford) 61:e267–e269. https://doi.org/10.1093/rheumatology/keac226
Kume K, Amano K, Yamada S et al (2017) Tofacitinib improves atherosclerosis despite up-regulating serum cholesterol in patients with active rheumatoid arthritis: a cohort study. Rheumatol Int 37:2079–2085. https://doi.org/10.1007/s00296-017-3844-9
Wu JJ, Strober BE, Hansen PR et al (2016) Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long-term extension data in patients with plaque psoriasis. J Am Acad Dermatol 75:897–905. https://doi.org/10.1016/j.jaad.2016.06.012
Wolk R, Armstrong EJ, Hansen PR et al (2017) Effect of tofacitinib on lipid levels and lipid-related parameters in patients with moderate to severe psoriasis. J Clin Lipidol 11:1243–1256. https://doi.org/10.1016/j.jacl.2017.06.012
Sleutjes JAM, Roeters van Lennep JE, van der Woude CJ, de Vries AC (2022) Lipid changes after induction therapy in patients with inflammatory bowel disease: effect of different drug classes and inflammation. Inflamm Bowel Dis. Online ahead of print. https://doi.org/10.1093/ibd/izac100
Sands BE, Taub PR, Armuzzi A et al (2020) Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol 18:123-132.e123. https://doi.org/10.1016/j.cgh.2019.04.059
Ritchlin CT, Giles JT, Ogdie A et al (2020) Tofacitinib in patients with psoriatic arthritis and metabolic syndrome: a post hoc analysis of phase 3 studies. ACR Open Rheumatol 2:543–554. https://doi.org/10.1002/acr2.11166
Kim J, Tomalin L, Lee J et al (2018) Reduction of inflammatory and cardiovascular proteins in the blood of patients with psoriasis: differential responses between tofacitinib and etanercept after 4 weeks of treatment. J Invest Dermatol 138:273–281. https://doi.org/10.1016/j.jid.2017.08.040
Totoson P, Peyronnel C, Quirié A et al (2022) Tofacitinib improved peripheral endothelial dysfunction and brain-derived neurotrophic factor levels in the rat adjuvant-induced arthritis model. Fundam Clin Pharmacol 36:363–374. https://doi.org/10.1111/fcp.12731
Baldini C, Moriconi FR, Galimberti S, Libby P, De Caterina R (2021) The JAK-STAT pathway: an emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur Heart J 42:4389–4400. https://doi.org/10.1093/eurheartj/ehab447
Taylor PC, Weinblatt ME, Burmester GR et al (2019) Cardiovascular Safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol 71:1042–1055. https://doi.org/10.1002/art.40841
Qiu C, Zhao X, She L et al (2019) Baricitinib induces LDL-C and HDL-C increases in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Lipids Health Dis 18:54. https://doi.org/10.1186/s12944-019-0994-7
Taylor PC, Takeuchi T, Burmester GR et al (2022) Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis 81:335–343. https://doi.org/10.1136/annrheumdis-2021-221276
Huang F, Luo ZC (2018) Risk of adverse drug events observed with baricitinib 2 mg versus baricitinib 4 mg once daily for the treatment of rheumatoid arthritis: a systematic review and meta-analysis of randomized controlled trials. BioDrugs 32:415–423. https://doi.org/10.1007/s40259-018-0304-3
US FDA prescribing information for baricitinib. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s006lbl.pdf. Accessed 19 Sept 2022
Conaghan PG, Mysler E, Tanaka Y et al (2021) Upadacitinib in rheumatoid arthritis: a benefit-risk assessment across a phase III program. Drug Saf 44:515–530. https://doi.org/10.1007/s40264-020-01036-w
Cohen SB, van Vollenhoven RF, Winthrop KL et al (2021) Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis 80:304–311. https://doi.org/10.1136/annrheumdis-2020-218510
Fleischmann R, Mysler E, Bessette L et al (2022) Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: results through 3 years from the SELECT-COMPARE study. RMD Open 8:e002012. https://doi.org/10.1136/rmdopen-2021-002012
Zeng X, Zhao D, Radominski SC et al (2021) Upadacitinib in patients from China, Brazil, and South Korea with rheumatoid arthritis and an inadequate response to conventional therapy. Int J Rheum Dis 24:1530–1539. https://doi.org/10.1111/1756-185x.14235
Burmester GR, Winthrop K, Blanco R et al (2022) Safety profile of upadacitinib up to 3 years in psoriatic arthritis: an integrated analysis of two pivotal phase 3 trials. Rheumatol Ther 9:521–539. https://doi.org/10.1007/s40744-021-00410-z
Yamaoka K, Tanaka Y, Kameda H et al (2021) The Safety Profile of Upadacitinib in Patients with Rheumatoid Arthritis in Japan. Drug Saf 44:711–722. https://doi.org/10.1007/s40264-021-01067-x
Xie W, Huang Y, Xiao S, Sun X, Fan Y, Zhang Z (2019) Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis 78:1048–1054. https://doi.org/10.1136/annrheumdis-2018-214846
Xie W, Xiao S, Huang Y, Sun X, Zhang Z (2019) Effect of tofacitinib on cardiovascular events and all-cause mortality in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis 11:1759720x198954925. https://doi.org/10.1177/1759720x19895492
Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L (2020) Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology 158:1554-1573.e1512. https://doi.org/10.1053/j.gastro.2020.01.001
Yates M, Mootoo A, Adas M et al (2021) Venous thromboembolism risk with JAK inhibitors: a meta-analysis. Arthritis Rheumatol 73:779–788. https://doi.org/10.1002/art.41580
Alves C, Penedones A, Mendes D, Marques FB (2022) Risk of cardiovascular and venous thromboembolic events associated with Janus kinase inhibitors in rheumatoid arthritis: a systematic review and network meta-analysis. J Clin Rheumatol 28:69–76. https://doi.org/10.1097/rhu.0000000000001804
Dougados M, Charles-Schoeman C, Szekanecz Z et al (2022) OP0264 Impact of baseline cardiovascular risk on the incidence of major adverse cardiovascular events in the tofacitinib rheumatoid arthritis clinical programme. Ann Rheum Dis 81:175. https://doi.org/10.1136/annrheumdis-2022-eular.879
Szekanecz Z, Giles JT, Buch MH et al (2022) POS0110 Incidence of major adverse cardiovascular events stratified by geographic region and baseline cardiovascular risk: a post hoc analysis of oral surveillance. Ann Rheum Dis 81:278. https://doi.org/10.1136/annrheumdis-2022-eular.1180
Buch MH, Charles-Schoeman C, Curtis J et al (2022) POS0237 Major adverse cardiovascular events, malignancies and venous thromboembolism by baseline cardiovascular risk: a post hoc analysis of oral surveillance. Ann Rheum Dis 81:356. https://doi.org/10.1136/annrheumdis-2022-eular.1182
Charles-Schoeman C, Buch MH, Dougados M et al (2022) POS0674 Risk factors for major adverse cardiovascular events in patients aged ≥50 years with rheumatoid arthritis and ≥1 additional cardiovascular risk factor: a post hoc analysis of oral surveillance. Ann Rheum Dis 81:611. https://doi.org/10.1136/annrheumdis-2022-eular.1234
Giles JT, Charles-Schoeman C, Buch MH et al (2022) POS0520 Association between baseline statin treatment and major adverse cardiovascular events in patients with rheumatoid arthritis: a post hoc analysis of oral surveillance. Ann Rheum Dis 81:518. https://doi.org/10.1136/annrheumdis-2022-eular.1255
Karpouzas G, Szekanecz Z, Baecklund E et al (2022) POS0519 Relationship between disease activity and major adverse events in patients with rheumatoid arthritis on tofacitinib or TNF inhibitors: a post hoc analysis of oral surveillance. Ann Rheum Dis 81:517. https://doi.org/10.1136/annrheumdis-2022-eular.1238
Charles-Schoeman C, Fleischmann RM, Mysler E et al (2022) POS0239 Risk of venous thromboembolic events in patients with rheumatoid arthritis aged ≥50 years with ≥1 cardiovascular risk factor: results from a phase 3b/4 randomised study of tofacitinib vs tumour necrosis factor inhibitors. Ann Rheum Dis 81:358. https://doi.org/10.1136/annrheumdis-2022-eular.1016
Szekanecz Z, Charles-Schoeman C, Vranic I et al (2022) OP0269 Biomarkers to predict risk of venous thromboembolism in patients with rheumatoid arthritis receiving tofacitinib or tumour necrosis factor inhibitors. Ann Rheum Dis 81:179. https://doi.org/10.1136/annrheumdis-2022-eular.787
Qian J, Xue X, Shannon J (2022) Characteristics of adverse event reporting of Xeljanz/Xeljanz XR, Olumiant, and Rinvoq to the US Food and Drug Administration. J Manag Care Spec Pharm 28:1046–1052. https://doi.org/10.18553/jmcp.2022.28.9.1046
Author information
Authors and Affiliations
Contributions
The conception and design of the study – DPM, VA.
Acquisition of data, analysis and interpretation of data – DPM, GP.
Drafting the article – DPM, GP.
Revising it critically for important intellectual content – VA.
Final approval of the version to be submitted – DPM, GP, VA.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved – DPM, GP, VA.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Durga Prasanna Misra declares that he has no conflict of interest.
Gaurav Pande declares that he has no conflict of interest.
Vikas Agarwal declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Misra, D.P., Pande, G. & Agarwal, V. Cardiovascular risks associated with Janus kinase inhibitors: peering outside the black box. Clin Rheumatol 42, 621–632 (2023). https://doi.org/10.1007/s10067-022-06415-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06415-5