Abstract
Background
This meta-analysis aims to determine the association between antibodies including anti-citrullinated protein antibodies (ACPA) and rheumatoid factors (RF) and risk of rheumatoid arthritis–related interstitial lung disease (RA-ILD).
Methods
PubMed, Embase, and Cochrane were searched up to September 13, 2020, for studies investigating the risk of RA-ILD in ACPA-positive patients. The statistical meta-analysis and sensitivity analysis were performed using the Review Manager 5.4 and Stata16.0 software, respectively.
Results
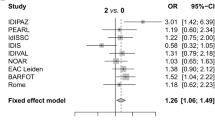
Total 1 double-blind randomized controlled study and 16 observational studies, including 992 RA-ILD patients and 2223 RA-non ILD patients, met the inclusion criteria of the meta-analysis. Compared with ACPA-negative patients, positive serum ACPA increased the risk of RA-ILD (OR = 2.51; 95% CI: 1.35–4.68; P = 0.004) and serum ACPA titer was significantly correlated with risk of RA-ILD (SMD = 0.39; 95% CI: 0.17–0.62; P = 0.0006). In a region-based subgroup analysis, ACPA titer in Asian, European, and African populations was significantly related to the risk of RA-ILD, while there was no significant correlation in the Americans (SMD = − 0.03; 95% CI: − 0.89–0.83; P = 0.95), especially in the USA (SMD = 0.37; 95% CI: − 0.26–0.99; P = 0.25). In addition, serum positive RF increased the risk of RA-ILD (OR = 2.85; 95% CI: 2.19–3.71; P < 0.00001) and serum RF titer was significantly correlated with the risk of RA-ILD (SMD = 0.35; 95% CI: 0.23–0.46; P < 0.00001). However, for the analysis of RF dichotomous data, the funnel shape was asymmetric and the p value of egger test was less than 0.05, which indicated potential publication bias.
Conclusions
ACPA and RF positive patients have greater risk of RA-ILD, and RA patients positive for ACPA should be paid more attention.
Key Points
• Autoantibodies ACPA and RF increase the risk of RA-ILD.
• Regions may be related to RA-ILD.





Similar content being viewed by others
References
Huizinga TW, Pincus T (2010) In the clinic. Rheumatoid arthritis. Annals of internal medicine 153 (1):ITC1–1-ITC1–15; quiz ITC11–16. https://doi.org/10.7326/0003-4819-153-1-201007060-01001
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581. https://doi.org/10.1002/art.27584
Demoruelle MK, Parish MC, Derber LA, Kolfenbach JR, Hughes-Austin JM, Weisman MH, Gilliland W, Edison JD, Buckner JH, Mikuls TR, O’Dell JR, Keating RM, Gregersen PK, Norris JM, Holers VM, Deane KD (2013) Performance of anti-cyclic citrullinated peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease. Arthritis Rheum 65(9):2243–2252. https://doi.org/10.1002/art.38017
Tsuchiya Y, Takayanagi N, Sugiura H, Miyahara Y, Tokunaga D, Kawabata Y, Sugita Y (2011) Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J 37(6):1411–1417. https://doi.org/10.1183/09031936.00019210
Lee DM, Schur PH (2003) Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis 62(9):870–874. https://doi.org/10.1136/ard.62.9.870
Nell VP, Machold KP, Stamm TA, Eberl G, Heinzl H, Uffmann M, Smolen JS, Steiner G (2005) Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis 64(12):1731–1736. https://doi.org/10.1136/ard.2005.035691
Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, Engström M, Grunewald J, Nyren S, Eklund A, Klareskog L, Sköld CM, Catrina AI (2014) Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis & rheumatology (Hoboken, NJ) 66(1):31–39. https://doi.org/10.1002/art.38201
Aubart F, Crestani B, Nicaise-Roland P, Tubach F, Bollet C, Dawidowicz K, Quintin E, Hayem G, Palazzo E, Meyer O, Chollet-Martin S, Dieudé P (2011) High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J Rheumatol 38(6):979–982. https://doi.org/10.3899/jrheum.101261
Inui N, Enomoto N, Suda T, Kageyama Y, Watanabe H, Chida K (2008) Anti-cyclic citrullinated peptide antibodies in lung diseases associated with rheumatoid arthritis. Clin Biochem 41(13):1074–1077. https://doi.org/10.1016/j.clinbiochem.2008.06.014
Zhu J, Zhou Y, Chen X, Li J (2014) A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J Rheumatol 41(7):1282–1289. https://doi.org/10.3899/jrheum.131341
Fan W, Chen J, Bao C (2016) An updated meta-analysis of association of rheumatoid arthritis related interstitial lung disease with anti-cyclic citrullinated peptide antibody or rheumatoid factor. Int J Clin Exp Med 9(6):10333–10343
Restrepo JF, del Rincón I, Battafarano DF, Haas RW, Doria M, Escalante A (2015) Clinical and laboratory factors associated with interstitial lung disease in rheumatoid arthritis. Clin Rheumatol 34(9):1529–1536. https://doi.org/10.1007/s10067-015-3025-8
Wang JX, Du CG (2015) A retrospective study of clinical characteristics of interstitial lung disease associated with rheumatoid arthritis in Chinese patients. Med Sci Monit 21:708–715. https://doi.org/10.12659/msm.890880
Song ST, Kim SS, Kim JY, Lee SY, Kim K, Kwon IS, Kim JN, Park WH, Yoo IS, Yoo SJ, Kim JH, Kang SW, Shim SC (2016) Association of single nucleotide polymorphisms of PADI4 and HLA-DRB1 alleles with susceptibility to rheumatoid arthritis-related lung diseases. Lung 194(5):745–753. https://doi.org/10.1007/s00408-016-9916-x
Chen MZ, Fu Q, Fu Q, Yang CD, Li T (2016) Study on clinical characteristics and risk factors of interstitial lung disease in 117 patients with rheumatoid arthritis. Journal of Shanghai Jiaotong University (Medical Science) 36 (3):359–363 and 384. https://doi.org/10.3969/j.issn.1674-8115.2016.03.009
Zhang LL, Wu LJ (2016) Clinical and laboratory correlation study of rheumatoid arthritis associated interstitial lung disease. Int J Rheum Dis 19:116. https://doi.org/10.1111/1756-185X.12962
Fadda S, Khairy N, Fayed H, Mousa H, Taha R (2018) Interstitial lung disease in Egyptian patients with rheumatoid arthritis: frequency, pattern and correlation with clinical manifestations and anti-citrullinated peptide antibodies level. Egyptian Rheumatologist 40(3):155–160. https://doi.org/10.1016/j.ejr.2017.10.006
Zhu H, Zhao LJ, Zhou Y, Chen Y (2019) [Significance of anti-carbamylated protein antibodies in patients with rheumatoid arthritis-associated intersitial lung disease]. Beijing da xue xue bao Yi xue ban = Journal of Peking University Health sciences 51 (6):1003–1007. https://doi.org/10.19723/j.issn.1671-167X.2019.06.004
Salaffi F, Carotti M, Di Carlo M, Tardella M, Giovagnoni A (2019) High-resolution computed tomography of the lung in patients with rheumatoid arthritis: prevalence of interstitial lung disease involvement and determinants of abnormalities. Medicine 98(38):e17088. https://doi.org/10.1097/md.0000000000017088
Dong H, Julien PJ, Demoruelle MK, Deane KD, Weisman MH (2019) Interstitial lung abnormalities in patients with early rheumatoid arthritis: a pilot study evaluating prevalence and progression. European journal of rheumatology 6(4):193–198. https://doi.org/10.5152/eurjrheum.2019.19044
Wu X, Xu L, Cheng Q, Nie L, Zhang S, Du Y, Xue J (2020) Increased serum soluble programmed death ligand 1(sPD-L1) is associated with the presence of interstitial lung disease in rheumatoid arthritis: a monocentric cross-sectional study. Respir Med 166:105948. https://doi.org/10.1016/j.rmed.2020.105948
Del Angel-Pablo AD, Buendía-Roldán I, Mejía M, Pérez-Rubio G, Nava-Quiroz KJ, Rojas-Serrano J, Falfán-Valencia R (2020) Anti-HLA class II antibodies correlate with C-reactive protein levels in patients with rheumatoid arthritis associated with interstitial lung disease. Cells 9 (3). https://doi.org/10.3390/cells9030691
Castellanos-Moreira R, Rodríguez-García SC, Gomara MJ, Ruiz-Esquide V, Cuervo A, Casafont-Solé I, Ramírez J, Holgado S, Gómez-Puerta JA, Cañete JD, Haro I, Sanmarti R (2020) Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: evidence of a new autoantibody linked to interstitial lung disease. Ann Rheum Dis 79(5):587–594. https://doi.org/10.1136/annrheumdis-2019-216709
Wang N, Zhang Q, Jing X, Guo J, Huang H, Xu Z (2020) The association between MUC5B mutations and clinical outcome in patients with rheumatoid arthritis-associated interstitial lung disease: a retrospective exploratory study in China. Med Sci Monit 26:e920137. https://doi.org/10.12659/msm.920137
Allam A, Youssef S, Moussa H, Ezzat Y (2020) Anti-citrullinated peptide antibodies with interstitial lung disease in patients with rheumatoid arthritis. Egypt J Chest Dis Tuberc 69(1):171–177. https://doi.org/10.4103/ejcdt.ejcdt_102_19
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 339:b2535. https://doi.org/10.1136/bmj.b2535
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324. https://doi.org/10.1002/art.1780310302
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69(9):1580–1588. https://doi.org/10.1136/ard.2010.138461
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, McNeil J, Moher D, Mack D, Patel D (2004) Celiac disease. Evid Rep Technol Assess (Summ) 104:1–6
Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L (2015) The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8(1):2–10. https://doi.org/10.1111/jebm.12141
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Giles JT, Danoff SK, Sokolove J, Wagner CA, Winchester R, Pappas DA, Siegelman S, Connors G, Robinson WH, Bathon JM (2014) Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis 73(8):1487–1494. https://doi.org/10.1136/annrheumdis-2012-203160
Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, Dawson J, Sathi N, Ahmad Y, Koduri G, Young A (2014) Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatology (Oxford) 53(9):1676–1682. https://doi.org/10.1093/rheumatology/keu165
Spagnolo P, Grunewald J, du Bois RM (2014) Genetic determinants of pulmonary fibrosis: evolving concepts. Lancet Respir Med 2(5):416–428. https://doi.org/10.1016/s2213-2600(14)70047-5
Paulin F, Babini A, Mamani M, Mercado J, Caro F (2017) Practical approach to the evaluation and management of rheumatoid arthritis-interstitial lung disease based on its proven and hypothetical mechanisms. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion 69 (5):235–242. https://doi.org/10.24875/ric.17002162
Spagnolo P, Lee JS, Sverzellati N, Rossi G, Cottin V (2018) The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheumatol (Hoboken, NJ) 70(10):1544–1554. https://doi.org/10.1002/art.40574
Funding
This study was funded by the Hunan Provincial Health Committee 225 Talent Project and funded by the Project of Changsha Science and Technology Bureau, kq1901119.
Author information
Authors and Affiliations
Contributions
FL and SSX conceived and designed the study. BLC and QZ conducted the literature search. SL and LCX conducted the data extraction. SSX conducted meta-analysis and interpreted the data. SSX and SL wrote the draft of the manuscript. FL and SSX critically revised the manuscript. All authors approved submission of the final version of the manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was not required since no patients participated in the study.
Research involving human participants and/or animals
For this type of study, formal consent is not required.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sisi Xie and Shu Li1 are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, S., Li, S., Chen, B. et al. Serum anti-citrullinated protein antibodies and rheumatoid factor increase the risk of rheumatoid arthritis–related interstitial lung disease: a meta-analysis. Clin Rheumatol 40, 4533–4543 (2021). https://doi.org/10.1007/s10067-021-05808-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05808-2