Abstract
Objectives
To compare the safety, efficacy, and immunogenicity of MSB11022 (acetate-buffered formulation), an adalimumab biosimilar, with the reference product.
Method
AURIEL-RA study was a phase 3, multicenter, randomized, double-blind, parallel group trial (NCT03052322). Patients with moderately-to-severely active rheumatoid arthritis (RA) with an inadequate response to methotrexate were randomized 1:1 to MSB11022 or reference adalimumab. The primary endpoint was the incidence of treatment-emergent adverse events of special interest (AESIs) (predefined as hypersensitivity) up to week 52. The key secondary endpoint was ACR20 (≥ 20% improvement in American College of Rheumatology core set measurements from baseline) at week 12. Other efficacy endpoints, quality of life, immunogenicity, and pharmacokinetic parameters were evaluated up to week 52. Secondary safety endpoints were evaluated up to week 52 and at a 4-month safety follow-up.
Results
In total, 288 patients were randomized. The proportion of patients experiencing ≥ 1 treatment-emergent AESI up to week 52 was similar between trial arms: 6 patients (4.2%; 95% CI 1.56, 8.91) receiving MSB11022, and 8 patients (5.5%; 95% CI 2.41, 10.58) receiving reference adalimumab. No clinically meaningful differences in efficacy, quality of life, or immunogenicity were seen between treatment arms up to week 52. No notable difference in the incidence of treatment-emergent adverse events was observed between treatment arms up to the end of the follow-up period.
Conclusions
These results suggest MSB11022 and reference adalimumab are similar in patients with moderately-to-severely active rheumatoid arthritis in terms of safety, immunogenicity, and efficacy. AURIEL-RA provides evidence to support the similarity of MSB11022 and adalimumab.
Key Points • Incidences of hypersensitivity events were similar for MSB11022 (modified buffer) and reference adalimumab. • There was no difference in local reactions between MSB11022 (modified buffer) and reference adalimumab. • AURIEL-RA confirms the equivalence in efficacy and immunogenicity of MSB11022 (modified buffer) and reference adalimumab. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation of peripheral joints. Irreversible cartilage and bone destruction can occur, leading to pain, disability, and functional decline [1, 2]. Biologics, such as the tumor necrosis factor (TNF) alpha inhibitor adalimumab, have been shown to have significant efficacy in RA, including improvements in disease activity and quality of life (QoL), and in slowing radiographic progression of structural damage to joints [3,4,5]. However, biologics are expensive, and globally, many patients do not have access to these treatments [6].
The introduction of biosimilars has been shown to reduce healthcare costs and may enable more patients to be treated within the same budget constraints [7, 8]. In the treatment of RA, this is now reflected in international guidelines, such as those provided by the European League Against Rheumatism (EULAR), which recommend the use of cost-effective treatment approaches as long as safety and outcomes are similar and in line with established therapeutic paradigms [9].
MSB11022 is a proposed adalimumab biosimilar that has been shown to be structurally and functionally similar to the reference adalimumab molecule based on an extensive analytical characterization exercise [10]. MSB11022 has been developed using two formulations: one in a citrate-based buffer, and one in an acetate-based buffer. Both contain the same pharmacologically active molecule. In a phase 1, double-blind study in healthy volunteers, bioequivalence and comparable safety, tolerability, and immunogenicity profiles were demonstrated between MSB11022 and reference adalimumab (both in a citrate-based buffer) [11]. Therapeutic equivalence between MSB11022 and reference adalimumab (both in a citrate-based buffer) in terms of efficacy, safety, and immunogenicity was shown in the pivotal AURIEL-PsO study. In patients with moderate-to-severe chronic plaque psoriasis, the Psoriasis Area and Severity Index (PASI) 75 response rate and the mean improvement in PASI were similar between the trial arms. QoL scores and safety and immunogenicity profiles were also similar [12]. In an additional healthy volunteer study (EMR200588-003, unpublished data), MSB11022 (acetate formulation) demonstrated bioequivalence and a comparable safety and immunogenicity profile to MSB11022 (citrate formulation).
Here we report the results from the AURIEL-RA study designed to compare the safety, immunogenicity, and efficacy of MSB11022 (acetate formulation) with reference adalimumab (citrate formulation) in patients with moderately-to-severely active RA with an inadequate response to methotrexate.
Methods
Study design
The AURIEL-RA study (NCT03052322; EudraCT 2016-002852-26) was a phase 3 multicenter, randomized, double-blind parallel group trial conducted from 31 January 2017 to 7 August 2018. The study was conducted in 47 centers across 6 European countries. The trial consisted of 3 periods: a 4-week screening period, a 52-week double-blind treatment period, and a 4-month safety follow-up period. Following the screening period, patients were randomized 1:1 in permuted blocks by a central interactive web response system to receive MSB11022 (acetate formulation) or reference adalimumab (Humira® citrate formulation; AbbVie Inc., North Chicago, IL, USA; European Union authorized). Patients were stratified by previous systemic therapy use: non-biologic versus biologic. Patients who previously received both biologic and non-biologic systemic therapies were assigned to the “biologic” group. The participation of patients in the previous biologic systemic therapy stratum was capped at 20% of the total number of patients randomized. The trial was double-blinded and blinding was maintained throughout the trial. Emergency unblinding did not occur. MSB11022 or reference adalimumab was administered at a dose of 40 mg subcutaneously every other week starting at baseline, up to, and including week 48.
Patients who achieved less than 20% improvement in both swollen and tender joint counts at week 24 or at any scheduled visit from week 24 up to week 52 discontinued study treatment (but participated in the safety and immunogenicity assessments).
Study population
Patients aged ≥ 18 years with a clinical diagnosis of moderately-to-severely active RA with disease duration ≥ 6 months from confirmed diagnosis were eligible for inclusion (defined by the 2010 revised American College of Rheumatology (ACR)/EULAR criteria or the 1987 Criteria of the American Rheumatology Association) [13, 14]. Patients were required to have ≥ 6 swollen joints and ≥ 6 tender joints (from the 66/68 joint count system) at screening and randomization, and either erythrocyte sedimentation rate (ESR) ≥ 28 mm/h or serum C-reactive protein (CRP) ≥ 1.0 mg/dl at screening. Patients were treated with methotrexate for ≥ 12 weeks prior to baseline with both a stable route of administration (oral or parenteral) and stable dose (10–25 mg/week) for ≥ 4 weeks prior to screening. Patients were required to be adalimumab-naïve and to have discontinued infliximab, certolizumab pegol, or golimumab at least 8 weeks prior to screening. Etanercept had to be discontinued 4 weeks prior to screening. Exposure to previous biologics was limited to one TNFα inhibitor other than adalimumab. Patients had to have no history of tuberculosis, no active tuberculosis, and no history or evidence of latent tuberculosis. Detailed exclusion criteria and prohibited medications are listed in Online Resource 2.
All subjects provided written informed consent. The study was conducted in accordance with the current International Council for Harmonisation Good Clinical Practice and the Declaration of Helsinki as well as with applicable local regulations.
Assessments
The primary endpoint was the incidence of treatment-emergent adverse events of special interest (AESIs) (predefined as hypersensitivity according to Standardized MedDRA Queries [narrow] as per the latest MedDRA version) up to and including week 52. The key secondary endpoint was ACR20 at week 12 (defined as improvement of ≥ 20% from baseline in the ACR core set of measurements) [15]. Other secondary efficacy endpoints included ACR20 at weeks 2, 4, 8, and 52, and assessments of ACR50, ACR70, Disease Activity Score 28-joint count-ESR (DAS28-ESR), Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI), and ACR/EULAR Boolean remission rates at weeks 2, 4, 8, 12, 24, and 52. QoL was assessed using the Disability Index (HAQ-DI) [16], 36-item Short Form Health Survey (SF-36) [17, 18], and Euro Quality of Life – 5 dimensions and 5 levels (EQ-5D-5L) [19] questionnaires at baseline and weeks 2, 4, 8, 12, 24, and 52 (HAQ-DI only at weeks 2, 4, and 8).
Antidrug antibodies (ADAs) and neutralizing antibodies (NAbs) were assessed at baseline and at scheduled visits at weeks 2, 4, 12, 24, 36, and 52. Blood samples were taken pre-dose and serum concentrations were quantified using a validated enzyme-linked immunosorbent assay. The incidence of ADAs was assessed using a highly sensitive and drug-tolerant validated bioanalytical method based on the Meso Scale Discovery Electrochemiluminescent platform (Meso Scale Diagnostics, Rockville, MD, USA). The assay sensitivity was 86.4 ng/mL with a drug tolerance of 250 μg/mL at the low positive control level of 129.6 ng/mL. In addition, all patients had blood samples taken for population pharmacokinetic (PK) assessment pre-first dose, and then prior to dosing on weeks 2, 4, 12, 24, 36, and 52.
Secondary safety endpoints included the incidence of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs) up to week 52 and at the 4-month safety follow-up. Injection site pain was evaluated using a 0–100 visual analogue scale (VAS) after 3 consecutive administrations of study drug (weeks 4, 6, and 8). Pain was evaluated immediately post-injection, and at 15 min and 1 h post-injection.
Statistical analysis
The primary endpoint was evaluated using the safety analysis set, which consisted of all randomized patients who received at least one dose of study drug. The incidence of treatment-emergent AESIs and 95% CIs were calculated for each treatment group. 95% CIs for the difference in incidence between groups were also calculated for any observed clinical events of AESIs (hypersensitivity) observed in ≥ 3% of patients. Therefore, the study was descriptive, and a sample size of approximately 260 patients was chosen to ensure at least 100 patients remained in each treatment arm at week 52. According to scientific advice from the European Medicines Agency, this sample size is informative for a study primarily focusing on safety in a biosimilar setting [20]. As such, this study was not powered to demonstrate equivalence between MSB11022 and reference adalimumab, and there was no hypothesis tested to justify the sample size.
For the key secondary endpoint, ACR20 at week 12, 95% CIs were calculated for each treatment group. This analysis was performed in both the per-protocol (PP; all randomized and treated patients without important protocol deviations) and intention-to-treat (ITT; all randomized patients) sets. Other secondary endpoints, including efficacy and QoL endpoints, were assessed using the PP and ITT sets, and were summarized descriptively according to the type of outcome. Immunogenicity and safety data, including injection site pain, were summarized descriptively using the safety analysis set. PK data were summarized using the PK analysis set. Analyses exploring associations between PK and immunogenicity and between PK and efficacy also used the PK analysis set.
Results
Disposition and demographics
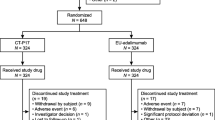
A total of 288 patients were randomized (ITT: MSB11022, n = 143; reference adalimumab, n = 145). (Fig. 1). All randomized patients received at least one dose of study drug and were included in the safety analysis set. Seven patients (4.9%) in the MSB11022 arm and 12 patients (8.3%) in the reference adalimumab arm were excluded from the PP analysis (PP: MSB11022, n = 136; reference adalimumab, n = 133). Reasons for exclusion from the PP set included being assigned to an incorrect randomization stratum with respect to systemic therapy previously received, using prohibited medication(s) and/or therapies at any time during the study, being < 80% compliant to subcutaneous injection administration, ACR20 could not be calculated at week 12, compliance was below 80% up to week 10, and discontinuing treatment before week 12. Patient demographics and baseline characteristics were similar between treatment arms (Table 1). In both treatment arms, the median number of injections was 25 and the median duration of exposure was 50 weeks.
Primary endpoint
Few treatment-emergent AESIs of hypersensitivity were reported during the study. The proportions of patients who experienced ≥ 1 hypersensitivity AESI by week 52 was similar across treatment arms: 6 patients (4.2%; 95% CI 1.56, 8.91) in the MSB11022 arm and 8 patients (5.5%; 95% CI 2.41, 10.58) in the reference adalimumab arm (Fig. 2). The types of hypersensitivity reactions recorded were comparable between treatment arms (Table 2). Two hypersensitivity reactions qualified as SAEs: one event of dermatitis (grade 3) in the reference adalimumab arm, and one anaphylactic reaction (grade 4) in the MSB11022 arm, which was considered to be treatment-related. Both events occurred after the completion of the study treatment. The first patient was hospitalized with a generalized erythematous exfoliating dermatitis of the whole body with scalp involvement and secondary superinfection. After 10 days of treatment, the subject was discharged from hospital with significant resorption of the lesions with no new eruptions and improvement of pruritus-related symptoms. The second patient experienced dyspnea, dizziness, vertigo, and nausea 30 min after administration of last study dose of MSB11022. The event resolved with treatment (chloropyramine and methylprednisolone). No subjects permanently discontinued treatment or withdrew from the study due to hypersensitivity, and there were no deaths reported subsequent to a hypersensitivity reaction.
Efficacy endpoints
Key secondary endpoint
Efficacy data are presented for the ITT population, and similar results were observed in the PP population. The proportion of patients who achieved ACR20 at week 12 was similar between MSB11022 and reference adalimumab (MSB11022: n = 113 [79.6%]; reference adalimumab: n = 114 [80.9%]; 95% CI for treatment difference −10.55, 8.04). This similarity was maintained up to week 52 (Fig. 3a).
Secondary endpoint: ACR20/50/70 response (a) and mean (SD) DAS28-ESR score (b) by study week (ITT analysis set). ACR20/50/70 ≥ 20/50/70% improvement in American College of Rheumatology response; DAS28, Disease Activity Score 28-joint count; ESR, erythrocyte sedimentation rate; ITT, intention-to-treat; SD, standard deviation
Other efficacy endpoints
The ACR50 and ACR70 response rates (Fig. 3a), DAS28-ESR scores (Fig. 3b), and SDAI and CDAI scores (Online Resource 3) were also similar between the MSB11022 and reference adalimumab treatment arms at scheduled visits up to week 52 in the ITT population and PP populations.
Quality of life endpoints and pain scores
EQ-5D-5L index values, EQ-5D-5L VAS, SF-36 scores, and HAQ-DI scores were similar for MSB11022 versus reference adalimumab at weeks 24 and 52 (Online Resource 4).
Injection site pain scores were low in both treatment arms at week 4, and further decreased with subsequent injections in the ITT population (Online Resource 5). The majority of patients experienced a 0 or very low pain score: based on third quartile data, at least 75% of patients reported a score immediately post-injection of ≤ 6 (out of 100) at week 4, ≤ 3 at week 6, and ≤ 2 at week 8.
Immunogenicity and pharmacokinetics
During the 52-week treatment period, 80.4% and 71.7% of patients in the MSB11022 and reference adalimumab treatment arms had at least one positive ADA result, and 39.9% and 39.3% of patients in the MSB11022 and reference adalimumab arms, respectively, had a positive NAb result (Fig. 4). At week 12, the mean (SD) adalimumab trough level for MSB11022 and reference adalimumab was 8,490 (2,291) ng/mL and 7,570 (3,169) ng/mL, respectively, in ADA-negative patients, and 5,070 (3,657) ng/mL and 5,050 (3,514) ng/mL, respectively, in ADA-positive patients. No meaningful differences in ACR20 response rates were reported at scheduled visits up to week 52 when looking separately at ADA-positive and ADA-negative status (Online Resource 6a).
For MSB11022 and reference adalimumab, the mean trough serum concentrations at week 24 were 6,380 ng/mL and 6,550 ng/mL, and at week 52, these were 5,610 ng/mL and 5,330 ng/mL, respectively, with similar concentrations across groups at all time points up to week 52. The presence of ADA had an impact on adalimumab trough concentrations, where ADA-positive subjects had lower trough concentrations compared with ADA-negative subjects; this trend was similar for both MSB11022 and reference adalimumab (Online Resource 6b). The influence of ADA on PK parameters was similar across treatment groups. Lower mean trough concentrations were also observed in the ADA-positive subjects who tested NAb-positive compared with those who tested NAb-negative (data not shown).
Other safety endpoints
The incidence of TEAEs up to week 52 was similar between the treatment arms (MSB11022: n = 83 [58.0%]; reference adalimumab: n = 93 [64.1%]; Table 2. A lower proportion of patients in the MSB11022 compared with the reference adalimumab treatment arm permanently discontinued treatment due to TEAEs (4.2% vs 9.7%). In the 4-month safety follow-up, only 6 patients in each arm experienced a TEAE; none of which were considered to be treatment-related. Few patients experienced an SAE during the treatment period or during the 4-month safety follow-up (Table 2). There was no notable imbalance between the treatment arms.
There were three deaths reported in the study. One patient died of unknown causes prior to randomization. One patient died in the reference adalimumab arm during the treatment period following arteriosclerosis in the coronary artery and myocardial ischemia; this was considered to be unrelated to the treatment. One patient died of unknown causes in the reference adalimumab arm during the 4-month safety follow-up. This was also considered to be unrelated to the treatment.
Fewer injection site reactions (ISRs) were reported in the MSB11022 arm compared with the reference adalimumab arm, 13 patients (9.1%) reported 56 ISRs with MSB11022 and 33 patients (22.8%) reported 298 ISRs with reference adalimumab. A large proportion of reported ISRs concentrated on a small number of subjects experiencing multiple repeat ISRs. The most commonly reported ISRs were erythema, pruritus, and pain, which accounted for more than half of all reported events.
There was no notable pattern in terms of AESIs, TEAEs, or ISRs between the treatment arms with regard to ADA status or NAb status.
Conclusion
The results of this study indicate that MSB11022 (acetate formulation) and reference adalimumab are similar with regard to safety, immunogenicity, and efficacy in patients with moderately-to-severely active RA while on stable treatment with methotrexate. This patient population is the most robustly studied population in phase 3 studies of adalimumab in RA [21] and represents a large proportion of patients receiving adalimumab. RA is also an example of a sensitive setting to investigate potential differences in immunogenicity, given the immunogenicity observed with TNF inhibitors in RA [22]. These results add to the evidence of similarity between the molecule MSB11022 and reference adalimumab, as demonstrated previously in patients with psoriasis in the AURIEL-PsO study [12] and provide data for the acetate formulation in this disease population.
There were few treatment-emergent hypersensitivity reactions up to week 52, and no differences were observed between treatment arms. The incidence of TEAEs was comparable up to week 52 and at the 4-month follow-up across both treatment groups, and no new safety signals were observed. A trend towards reduced incidence of ISRs was observed with MSB11022 compared with the reference product. However, this result was skewed by the fact that a small number of patients experienced a high number of events.
The results for the efficacy endpoints consistently indicated similarity between MSB11022 and the reference product, with similar proportions of patients in the MSB11022 and reference adalimumab arms achieving ACR20 at week 12. This similarity was maintained at all scheduled visits up to week 52. Similar efficacy between MSB11022 and reference adalimumab in RA was further confirmed with DAS28-ESR, CDAI and SDAI scores, and also in evaluations of QoL. This supports the findings of the AURIEL-PsO study, which demonstrated that MSB11022 was clinically similar to reference adalimumab in terms of the efficacy endpoints [12].
An evaluation of immunogenicity is a key part of the clinical assessment of biosimilars. In this study, there were no clinically meaningful differences in the incidence of ADAs and NAbs between treatment arms up to week 52. This is consistent with the results reported in the AURIEL-PsO study [12]. Of note, the incidence of ADAs and NAbs was considerably higher than was reported in the pivotal studies for reference adalimumab [23]. This may reflect the use of more sensitive and drug-tolerant assays than were used in earlier studies [24] and is consistent with other recent adalimumab studies [25]. Similar to the AURIEL-PsO study, in which PASI75 was the primary efficacy parameter, no differences in efficacy as measured by ACR20 response rates were reported between ADA-positive and ADA-negative patients in AURIEL-RA. The difference in ACR20 response rate observed in patients stratified by ADA status is comparable (Online Resource 6a). This observation stands out when compared with published data [26, 27] and may be due to the highly sensitive and drug-tolerant assay used for detecting ADAs. While adalimumab mean trough levels in ADA-negative patients are higher than for ADA-positive patients in both arms in our study, published data have indicated that adalimumab trough levels of 5–8 μg/mL are sufficient to reach adequate clinical response in patients with RA, and clinical effect was observed even in patients with adalimumab concentrations as low as 3 μg/mL [28].
The main limitation of this study is that it is descriptive only and was not powered to show equivalence between MSB11022 and reference adalimumab for any endpoint. In general, safety and tolerability studies are not powered due to the statistical challenges in analyzing safety and tolerability events, which cannot all be anticipated when designing a trial. Therefore, the European Medicines Agency recommends that, in most trials, safety implications are best addressed by applying descriptive statistical methods to the data, supplemented by calculation of CIs wherever this aids interpretation [29]. Similar concerns around uncertainty apply to studying immunogenicity [30] and its effect on safety, PK parameters, and efficacy [31, 32]. In addition, no switch arm was included, so no data on the impact of switching from reference adalimumab to MSB11022 in RA are available.
In conclusion, MSB11022 and reference adalimumab are comparable in terms of safety, immunogenicity, and efficacy. Furthermore, the results of the AURIEL-RA study in patients with RA are consistent with the results of the AURIEL-PsO study in patients with psoriasis, despite differences in the formulations tested in the two studies. This supports the overall biosimilarity concept for the MSB11022 molecule.
Data availability
Some of the data in this study have been presented in the following poster: Edwards CJ, et al. Safety, immunogenicity and efficacy of the proposed biosimilar MSB11022 (acetate-buffered formulation) compared with adalimumab reference product in patients with moderately to severely active rheumatoid arthritis: AURIEL-RA, a randomised, double-blind, Phase III study. Poster FRI0088. EULAR. 12–15 June 2019. Madrid, Spain.
References
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108. https://doi.org/10.1016/S0140-6736(10)60826-4
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423(6937):356–361. https://doi.org/10.1038/nature01661
Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, Sharp J, Perez JL, Spencer-Green GT (2006) The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 54(1):26–37. https://doi.org/10.1002/art.21519
van der Heijde D, Breedveld FC, Kavanaugh A, Keystone EC, Landewe R, Patra K, Pangan AL (2010) Disease activity, physical function, and radiographic progression after longterm therapy with adalimumab plus methotrexate: 5-year results of PREMIER. J Rheumatol 37(11):2237–2246. https://doi.org/10.3899/jrheum.100208
Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, Fischkoff SA, Chartash EK (2004) Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 50(5):1400–1411. https://doi.org/10.1002/art.20217
Bergstra SA, Branco JC, Vega-Morales D, Salomon-Escoto K, Govind N, Allaart CF, Landewe RBM (2018) Inequity in access to bDMARD care and how it influences disease outcomes across countries worldwide: results from the METEOR-registry. Ann Rheum Dis:annrheumdis-2018-213289. https://doi.org/10.1136/annrheumdis-2018-213289
Lyman GH, Zon R, Harvey RD, Schilsky RL (2018) Rationale, opportunities, and reality of biosimilar medications. N Engl J Med 378(21):2036–2044. https://doi.org/10.1056/NEJMhle1800125
Pentek M, Zrubka Z, Gulacsi L (2017) The economic impact of biosimilars on chronic immune-mediated inflammatory diseases. Curr Pharm Des 23(44):6770–6778. https://doi.org/10.2174/1381612824666171129193708
Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, Aletaha D, Aringer M, Boers M, Buckley CD, Buttgereit F, Bykerk V, Cardiel M, Combe B, Cutolo M, van Eijk-Hustings Y, Emery P, Finckh A, Gabay C, Gomez-Reino J, Gossec L, Gottenberg JE, Hazes JMW, Huizinga T, Jani M, Karateev D, Kouloumas M, Kvien T, Li Z, Mariette X, McInnes I, Mysler E, Nash P, Pavelka K, Poor G, Richez C, van Riel P, Rubbert-Roth A, Saag K, da Silva J, Stamm T, Takeuchi T, Westhovens R, de Wit M, van der Heijde D (2017) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 76(6):960–977. https://doi.org/10.1136/annrheumdis-2016-210715
Magnenat L, Palmese A, Fremaux C, D'Amici F, Terlizzese M, Rossi M, Chevalet L (2017) Demonstration of physicochemical and functional similarity between the proposed biosimilar adalimumab MSB11022 and Humira®. MAbs 9(1):127–139. https://doi.org/10.1080/19420862.2016.1259046
Hyland E, Mant T, Vlachos P, Attkins N, Ullmann M, Roy S, Wagner V (2016) Comparison of the pharmacokinetics, safety, and immunogenicity of MSB11022, a biosimilar of adalimumab, with Humira® in healthy subjects. Br J Clin Pharmacol 82(4):983–993. https://doi.org/10.1111/bcp.13039
Hercogova J, Papp K, Edwards CJ, Chryok V, Halady T, Ullman M, Vlachos P (2018) A randomized double-blind trial comparing the efficacy, safety, and immunogenicity of MSB11022, a proposed biosimilar of adalimumab, versus adalimumab originator in patients with moderate-to-severe plaque psoriasis. J Am Acad Dermatol 79(3):AB21. https://doi.org/10.1016/j.jaad.2018.05.126
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581. https://doi.org/10.1002/art.27584
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324. https://doi.org/10.1002/art.1780310302
Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R Jr, Paulus H, Strand V et al (1995) American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 38(6):727–735. https://doi.org/10.1002/art.1780380602
Bruce B, Fries JF (2005) The health assessment questionnaire (HAQ). Clin Exp Rheumatol 23(5 Suppl 39):S14–S18
McHorney CA, Ware JE Jr, Raczek AE (1993) The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31(3):247–263
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30(6):473–483
Yfantopoulos J, Chantzaras A, Kontodimas S (2017) Assessment of the psychometric properties of the EQ-5D-3L and EQ-5D-5L instruments in psoriasis. Arch Dermatol Res 309(5):357–370. https://doi.org/10.1007/s00403-017-1743-2
European Medicines Agency (EMA) (2016) Recommendation EMA/CHMP/SAWP/200743/2016
Zhao S, Chadwick L, Mysler E, Moots RJ (2018) Review of biosimilar trials and data on adalimumab in rheumatoid arthritis. Curr Rheumatol Rep 20(10):57. https://doi.org/10.1007/s11926-018-0769-6
Kalden JR, Schulze-Koops H (2017) Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol 13(12):707–718. https://doi.org/10.1038/nrrheum.2017.187
Abbvie Inc (2019) Prescribing information: HUMIRA (adalimumab) injection, for subcutaneous use. http://www.rxabbvie.com/pdf/humira.pdf. Accessed March 2019
Song S, Yang L, Trepicchio WL, Wyant T (2016) Understanding the supersensitive anti-drug antibody assay: unexpected high anti-drug antibody incidence and its clinical relevance. J Immunol Res 2016:3072586–3072588. https://doi.org/10.1155/2016/3072586
Gorovits B, Baltrukonis DJ, Bhattacharya I, Birchler MA, Finco D, Sikkema D, Vincent MS, Lula S, Marshall L, Hickling TP (2018) Immunoassay methods used in clinical studies for the detection of anti-drug antibodies to adalimumab and infliximab. Clin Exp Immunol 192(3):348–365. https://doi.org/10.1111/cei.13112
Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, Dijkmans BA, Aarden L, Wolbink GJ (2011) Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305(14):1460–1468. https://doi.org/10.1001/jama.2011.406
Moots RJ, Xavier RM, Mok CC, Rahman MU, Tsai WC, Al-Maini MH, Pavelka K, Mahgoub E, Kotak S, Korth-Bradley J, Pedersen R, Mele L, Shen Q, Vlahos B (2017) The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: results from a multinational, real-world clinical practice, non-interventional study. PLoS One 12(4):e0175207. https://doi.org/10.1371/journal.pone.0175207
Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D, Aarden L, Rispens T, Wolbink G (2015) Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis 74(3):513–518. https://doi.org/10.1136/annrheumdis-2013-204172
European Medicines Agency (EMA) (1998) Note for guidance on statistical principles for clinical trials (CPMP/ICH/363/96). https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-9-statistical-principles-clinical-trials-step-5_en.pdf. Accessed March 2019
European Medicines Agency (EMA) (2015) Guideline on Immunogenicity assessment of biotechnology-derived therapeutic proteins (EMEA/CHMP/BMWP/14327/2006 Rev. 1). https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-immunogenicity-assessment-biotechnology-derived-therapeutic-proteins-revision-1_en.pdf. Accessed March 2019
Knezevic I, Kang HN, Thorpe R (2015) Immunogenicity assessment of monoclonal antibody products: a simulated case study correlating antibody induction with clinical outcomes. Biologicals 43(5):307–317. https://doi.org/10.1016/j.biologicals.2015.06.009
Shankar G, Arkin S, Cocea L, Devanarayan V, Kirshner S, Kromminga A, Quarmby V, Richards S, Schneider CK, Subramanyam M, Swanson S, Verthelyi D, Yim S, American Association of Pharmaceutical S (2014) Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J 16(4):658–673. https://doi.org/10.1208/s12248-014-9599-2
Acknowledgements
Medical writing support was provided by Stephanie Carter and Dan Hami of Arc, a Division of Spirit Medical Communications Group Limited, supported by Fresenius Kabi. The list of principal investigators from each of the sites is included in Online Resource 1. This study was supported by Merck. Fresenius Kabi acquired the asset from Merck KGaA in September 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Prior to commencement of the study at a given site, the Clinical Study Protocol was submitted together with its associated documents, to the responsible Independent Ethics Committee (IEC) or Institutional Review Board (IRB) for its favorable opinion or approval. Amendments to these documents were submitted to the concerned IEC or IRB before implementation of substantial changes. Relevant safety information was submitted to the IEC or IRB during the course of the study in accordance with national regulations and requirements. All subjects provided written informed consent. The study was conducted in accordance with the current International Council for Harmonisation Good Clinical Practice and the Declaration of Helsinki, as well as with applicable local regulations. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest disclosure statement
C.J.E. has received honoraria for attendance at advisory boards for AbbVie, Biogen, BMS, Celgene, Fresenius Kabi, GSK, Janssen, Lilly, Mundipharma, Roche, and Sanofi; and as a consultant for Anthera, Merck, and Samsung Bioepis; and has received grants as an investigator for AbbVie, Biogen, and Pfizer. J.M, M.U., and V.G. are employees of Fresenius Kabi SwissBioSim. V.C. is a former employee of Fresenius Kabi SwissBioSim. P.V. has no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 479 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Edwards, C.J., Monnet, J., Ullmann, M. et al. Safety of adalimumab biosimilar MSB11022 (acetate-buffered formulation) in patients with moderately-to-severely active rheumatoid arthritis. Clin Rheumatol 38, 3381–3390 (2019). https://doi.org/10.1007/s10067-019-04679-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04679-y