Abstract
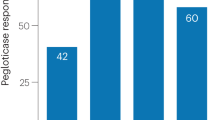
The objective of these clinical studies was to assess the safety and urate lowering activity of a novel urate transporter 1 (URAT1)/ xanthine oxidase (XO) inhibitor PF-06743649 in healthy subjects and gout patients. Escalating doses of PF-06743649 or placebo were given to healthy young subjects, healthy elderly subjects and gout patients. Serum uric acid (sUA) and urinary pharmacodynamic markers were assayed, and safety was assessed by collection of adverse events and assessment of safety labs, ECGs and vital signs. Administration of PF-06743649 led to rapid decrease in sUA in all cohorts; in gout patients, a change from baseline of 69 % was observed for the 40 mg dose. Urinary and serum biomarkers were consistent with inhibition of both URAT1 and XO. Although dosing was otherwise well tolerated, two subjects experienced serious adverse events of acute kidney injury. Both subjects exhibited increased serum creatinine and blood urea nitrogen in the first 3 days post first dose and were hospitalised. One subject exhibited oliguria for the first 24 h. Both subjects made a complete recovery with minimal intervention. PF-06743649 was effective at rapidly lowering sUA, but further development was terminated for an identified renal safety risk.


Similar content being viewed by others
References
Khanna D, FitzGerald JD, Khanna PP et al (2012) American college of rheumatology guidelines for management of gout part I: systematic non-pharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res 64(10):1431–1446. doi:10.1002/acr.21772
Watts RW (1966) Uric acid production with particular reference to the role of xanthine oxidase and its inhibition. Proc R Soc Med 59(4):287–292
Becker M, Schumacher H, Macdonald P, Lloyd E, Lademacher C (2009) Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. The J Rheumatol 36(6):1273–1282. doi:10.3899/jrheum.080814
Bridgeman M, Chavez B (2015) Febuxostat for the treatment of gout. Expert Opin Pharmacother 16(3):395–398. doi:10.1517/14656566.2015.985588
Bach M, Simkin P (2014) Uricosuric drugs. Curr Opin Rheumatol 26(2):169–175
ClinicalTrials.gov [Internet]. Identifier: NCT 02187029 Efficacy, safety and Tolerability of PF-06743649 in Gout Subjects. Available from: https://clinicaltrials.gov/ct2/show/NCT02187029
Shimizu K (2008) Japan.[Patent] WO2008126898A1.Available at: http://www.google.com.ar/patents/WO2008126898A1?cl = en
ClinicalTrials.gov [Internet]. Identifier: NCT 02151617 A study in Healthy people to Evaluate Saftey, Toleration, pharmacokinetics And Pharmacodynamics of multiple Oral Dose Of PF-06743649. Available from: https://clinicaltrials.gov/ct2/show/NCT02151617
ClinicalTrials.gov [Internet]. Identifier: NCT 02170012 A study in Healthy Elderly people to Evaluate Saftey, Toleration, pharmacokinetics And Pharmacodynamics of multiple Oral Dose Of PF-06743649. Available from: https://clinicaltrials.gov/ct2/show/NCT02170012
Wallance SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF (1977) Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 20(3):895–900
Fleischmann R, Kerr B, Yeh L, Suster M, Shen Z, Polvent E, Hingorani V, Quart B, Manhard K, Miner J, Baumgartner S (2014) Pharmacodynamic, pharmacokinetic and tolerability evaluation of concomitant administration of lesinurad and febuxostat in gout patients with hypercuricaemia. Rheumatology 53(12):2167–2174
Becker M, Schumacher H, Wortmann R, Schumacher R, MacDonald P, Eustace D, Palo W, Streit J, Ridge N-J (2005) Febuxostat compared with allopurinol in patients with hyperuricemia and gout. New Engl J Med 353:2450–2461. doi:10.1056/NEJMoa050373
Sawa H, Yamakawa H, Renjel L, Fernandes M (1980) Acute renal failure during treatment with ticrynafen. JAMA 243(8):766–767. doi:10.1001/jama.1980.03300340042018
NDA 207988: Lesinurad for the proposed indication of treatment of hyperuricemia associated with gout in combination with a xanthine oxidase inhibitor Edited by (FDA) FaDA. Arthritis Advisory Committee Meeting (2015) http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM467942.pdf
Lee MH, Graham GG, Williams KM, Ro D (2008) A benefit-risk assessment of benzbromarone in the treatment of gout. Was it withdrawal from the marker in the best interest of patients? Drug Saf 31(8):643–665
Acknowledgments
We thank the clinical trial sites involved in studies B7911001 (Pfizer Clinical Research Unit New Haven; PI Dan Rudin), B7911004 (Miami Research Associates: PI Martha Hernandez-Illas) and B7911002 (MRA: PI Martha Hernandez-Illas; Vince & Associates: Melodie Armstrong). We also thank John Hutchison and Santiago Arroyo (Pfizer) for advice during the planning and running of the studies.
The studies were funded by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All human studies were approved by the appropriate ethics committee and were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study.
Conflict of interest
Pinky Dua, Rachel Gurrell, Simon Kirby and Peter Loudon were all paid employees of Pfizer during the period of these studies. Maria Sudworth is an employee of Volt/AdClinico Consulting Limited, who was a paid consultant to Pfizer in connection with the development of this manuscript and worked as a clinician on this project.
Rights and permissions
About this article
Cite this article
Dua, P., Gurrell, R., Kirby, S. et al. Acute kidney injury observed during phase 1 clinical trials of a novel xanthine oxidase/URAT1 dual inhibitor PF-06743649. Clin Rheumatol 35, 2045–2051 (2016). https://doi.org/10.1007/s10067-016-3273-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3273-2